1. Introduction
Cardiovascular diseases (CVDs) have been the leading cause of death worldwide for many years [
1,
2]. It has been estimated that around 17.9 million lives are lost annually due to CVDs across the globe [
2]. Currently, there are no cures for any type of CVDs; there are only preventative measures to alleviate risk factors and in extreme cases perform heart transplants [
3,
4]. CVDs include a multitude of pathological conditions, including atherosclerosis, ischemic heart disease, stroke, and heart failure, to name a few [
5]. For this review, heart failure (HF) will be the focus. HF occurs due to structural or functional stress in the heart which no longer allows the heart to properly pump blood and oxygen to designated areas of the body [
6,
7,
8]. This is often manifested in decreased cardiac output and increased internal cardiac pressure [
7]. Annually, there are over 64 million cases of HF, accounting for over 346 billion US dollars, and these numbers are projected to rise [
9]. It is predicted that the rate of HF will increase by 50% in low and middle sociodemographic regions by 2030 [
9]. With no cure and increasing concern across the globe, there is a dire need to understand and treat this devastating disease.
Because of the lack of a cure for HF, many interventions have been utilized to attenuate risk factors. There is an assembly of drug-based interventions that have proven to be instrumental in treating the risk factors associated with HF, one of the most significant being chronic hypertension [
10]. Heart transplants do occur, but not as frequently as they are needed [
11]. To truly cure an injured heart, cardiac stem cells have been suggested and are currently being studied [
12]. However, they have not been as successful as originally proposed due to engraftment issues and detrimental immune responses [
13]. Alternatively, cardiac remodeling via epigenetic regulation has been studied and has had promising outcomes [
14]. In recent research, the combination of epigenetic regulated chromatin remodeling methods with the techniques, advancements, and theories of stem cell regulation have been groundbreaking for the field of cardiac biology [
15,
16].
The role of chromatin remodeling in HF is not a new idea, partially due to the overwhelming application of epigenetics in cardiac biology [
14,
17,
18,
19]. Generally, it is believed that when epigenetic modifications are altered there is a progression or suppression of the cardiac disease state [
20]. Normally, eukaryotic DNA is tightly condensed in chromatin, which is compacted around histones that form a nucleosome [
21]. These histones have all been identified (H1, H2A, H2B, H3, H4) and are well understood in cardiac biology [
22,
23,
24]. However, changes to these histones and the chromatin are less understood. Modifications such as a methylation and phosphorylation can be added to or removed from these structures, altering the chromatin architecture as well as the recruitment of complexes that can alter the overall chromatin structure [
25]. These alterations dictate whether the DNA becomes more or less tightly condensed. The more compacted the DNA, the less available the genes, meaning less gene expression or transcription.
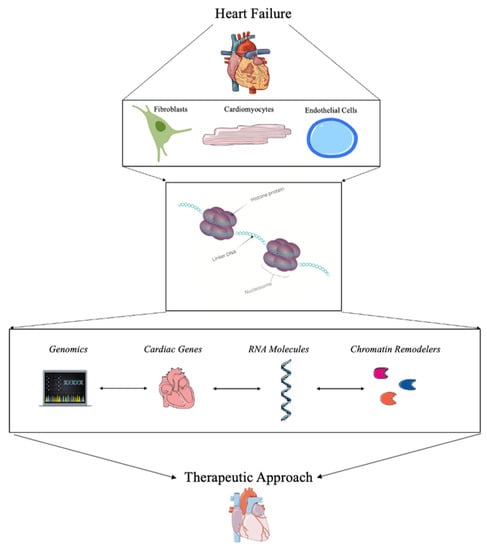
Targeting the changes in chromatin architecture or chromatin remodeling has been of interest over the past few years. With promising strategies, potential clinical trials, and the advancement of therapeutic approaches, chromatin remodeling in HF is a vital method for understanding this devasting disease to find a therapeutic approach to treating HF as depicted in Figure 1.
Figure 1. Summary of the role of chromatin remodeling for the treatment of heart failure.
2. Current Strategies for Detecting Chromatin Remodeling in Heart Failure
With advancements in bioinformatics as well as gene therapy technology, the ability to study chromatin as a clinical cardiac therapeutic has greatly increased over the last decade [
29,
30,
31,
32,
33]. Because chromatin is often tightly compacted and densely organized in the nucleus of cells, it can be difficult to isolate [
34]. Importantly, variations to the chromatin architecture are a three-dimensional, multifaceted challenge for researchers [
18]. To truly find a therapeutic approach, it is important to know the current challenges and new advancements in studying chromatin remodeling in the heart. Currently, there are standard approaches to studying changes in chromatin remodeling and gene expression due to changes in epigenetic profiles [
35,
36,
37]. Many of these approaches include a combination of genomics, RNA molecules, cardiac gene targets, and chromatin remodelers. Advancements in bioinformatics and gene therapy have proven to be pivotal in understanding pathological signaling in cardiac disease states; however, this can prove challenging and too broad for a true therapeutic approach. Additionally, targeting only a specific change in selective gene expression can be difficult and can cause other adverse downstream effects as well [
38]. However, a combination of all of these advancements could provide information to build a comprehensive understanding of chromatin remodeling during HF.
A more recent and promising strategy is using small molecules to target chromatin remodeling in the heart. By using chromatin remodeling enzymes, histone modifiers, chromatin regulatory complexes, and even DNA modifiers, the changes in chromatin architecture can be directly studied. This has been shown with the Switch/Sucrose-Nonfermentable (SWI/SNF) chromatin remodeling complex in many oncological studies, but it could also prove valuable in the heart [
39]. Similarly, small molecules such as long noncoding RNAs (lncRNAs) have been used to assess chromatin remodeling [
40]. The lncRNAs have been directly associated with structural changes in the chromatin in many disease models, including during pathological challenges to the heart [
28,
41]. One of the biggest advancements for understanding chromatin remodeling during HF is through genetic profiling approaches via sequencing and bioinformatic-based analysis. Using ATAC sequencing, RNA sequencing, and ChIP sequencing has led to major advancements in understanding epigenetic changes during HF. Specifically, for chromatin remodeling, combining these approaches in a novel “multiomic” approach has led to major discoveries that will be addressed in this review.
3. Therapeutic Approaches Using Chromatin Remodeling in Heart Failure
3.1. Genomics and Bioinformatics
The use of genomics to understand chromatin alterations and all the downstream changes has become a steadfast approach to most clinical applications of HF research. Many genomics-based research techniques are incorporated into most current studies along with other techniques, as this review will highlight. It is important to emphasize some of the most influential sequencing techniques that have made an impact on the field of chromatin remodeling in cardiac biology. One of the main methods to study chromatin remodeling through genomics is through the Assay for Transposase Accessible Chromatin (ATAC) sequencing. This ATAC sequencing data allows researchers to understand chromatin remodeling, chromatin accessibility, and epigenetic profiles [
37]. Through this sequencing method, studies have found pivotal transcriptional and chromatin accessibility differences in HF [
42]. For example, one study used ATAC sequencing to understand trans-aortic constriction in mice and found predictors of chromatin structural changes that led to a pivotal understanding of gene expression changes during disease progression [
43]. Another study used ATAC sequencing to analyze different genes found in hypoxia-induced stress associated with HF and found major changes in the chromatin’s accessibility during stress [
44]. ATAC sequencing is often paired with other genomic techniques or gene expression data.
Many of the widely used techniques include ATAC sequencing, RNA sequencing, miRNA sequencing, and DNA sequencing. All of these are not new techniques; however, how the data is used and interpreted is constantly changing. For example, one study used the multiomic approach, meaning they used single-cell RNA sequencing (scRNA seq), single-cell ATAC (scATAC seq), bulk ATAC sequencing, and miRNA sequencing to study HF in murine models [
45]. From these immense data sets, the authors found miRNA expression differences that aligned with chromatin accessibility profiles in mice with HF that were not seen in the control mice. All of this gave a new understanding of potential targets and mechanisms in the progression of HF [
45].
3.2. Targeting Cardiac Genes for Transcriptional Regulation
Chromatin remodeling in the heart is regulated by a multitude of genes. Some of these cardiac genes have been targeted during HF to assess changes in the cardiac disease state. For example, the transcription factor, Med1, was found to regulate chromatin remodeling in cardiomyocytes. Specifically, Med1 was found to synchronize the histone acetylation of lysine 27 (H3K27) which allowed for more open chromatin accessibility and therefore more gene expression [
50]. One study found that changes in gene expression in GATA4 and NXK2.5 were associated with changes in the chromatin architecture [
43]. Interestingly, the authors found a decrease in the protein CTCF, which is a chromatin structural protein, specifically after HF in murine cardiomyocytes [
43]. Likewise, another study found that GATA4 plays a major role in the chromatin structure, specifically in cardiac disease progression as well as cardiac development [
51].
It has also been found that changes in chromatin architecture have a role in cardiac fibroblast phenotypic gene expression. After cardiac injury, there is often an influx of cardiac fibroblasts that form scar tissue in the heart. Too much fibrosis can lead to irreversible damage and decreased heart function [
54]. It has been suggested that transcription factors such as GATA4, as well as MEF2C and TBX5, could be directly reprogrammed to alter their chromatin remodeling and therefore epigenetic signaling in fibrosis [
52,
53]. This has specifically been studied in cardiac fibroblasts in vitro and in vivo with high success [
55,
56,
57,
58,
59]. Additionally, changes in chromatic structure were shown to affect the stiffness of cardiac fibroblasts after injury [
60]. It was also found that topological change to cardiac fibroblasts significantly altered the chromatin remodeling and downstream cardiac gene expression associated with a decreased pathological response [
61]. The chromatin remodeling protein, BRG1, was also found to be a vital regulator of cardiac fibrosis, specifically in regulating the endothelial-to-mesenchymal transition [
62].
3.3. RNA Molecules
The use of small RNA molecules, often deemed non-coding RNA molecules, has been of great interest in epigenetic regulation and chromatin remodeling, specifically in HF [
64]. Many RNA molecules have been studied in pathological cardiac models, microRNAs (miRNAs), circular RNA (circRNA), and long non-coding RNAs (lncRNAs) being the primary focus due to influential and novel data [
65,
66,
67,
68].
It has been shown that the lncRNAs are regulated by ATP-dependent chromatin remodeling factors in both mouse and human hearts during HF [
40]. The lncRNAs have also been used as biomarkers for HF [
65,
66]. Current research has indicated that these lncRNAs may be the master regulators of disease progression in HF via chromatin remodeling [
69,
70,
71]. Many lncRNA can bind and regulate chromatin remodeling through epigenetic modifications and therefore regulate various cardiac gene expression levels during pathological stress [
61,
68].
The circular RNAs (circRNA) have also been of interest due to their ability to act as a sponge as well as a transcriptional regulator throughout cardiac tissue [
76]. Because of their unique role in cardiac tissue, circRNAs have been proposed as major biomarkers for HF [
77]. For example, one study found that the circRNA named circNCX1 played a major role in cardiomyocyte death during cardiac injury. Specifically, there was an increase in this circNCX1 with an increase in reactive oxygen species (ROS). The knockdown of this circRNA decreased the amount of cardiomyocyte cell death in mouse hearts [
78]. Often circRNAs do not act completely alone. One study found that the circRNA circ-HIPK3 interacted with the miRNA miR-17-3p to regulate calcium signaling during HF. Specifically, the downregulation of the circRNA seemed to lessen the fibrotic response and the progression of HF in adult mice [
79].
3.4. Chromatin Remodelers and Complexes
Unlike RNA molecules and cardiac gene transcription, chromatin remodelers play a direct role in regulating chromatin architecture. When the chromatin remodelers are dysregulated, the chromatin structure is altered, which can lead to devasting pathological responses that cause increased cardiac cell death, inflammatory signaling, and stress responses found in HF [
28]. One study found that the chromatin remodeling protein BRG1 and p300 had stage-specific regulation of histone acetylation. Specifically, these chromatin remodelers were upregulated during HF but not during left ventricular hypertrophy, indicating a step-wise transition regulated by chromatin regulators [
83]. The chromatin regulator, identified as SETD7, known for the methylation of histone 3 at lysine 4 (H3K4me1), was found to regulate inflammation pathways in obese patients with HF [
84]. It was found that the loss of SETD7 in murine cardiomyocytes protected against hypertrophy and further cardiac dysfunction that was directly associated with the regulation of inflammatory genes [
84]. Another study found that the interaction between a histone lysine methyltransferase named G9a and its downstream target Brain Derived Neurotrophic Factor (BDNF) regulated histone epigenetic modifications, therefore altering chromatin remodeling. It was found that G9a, which inhibits BDNF and increases cardiomyocyte death, is overexpressed in HF [
85]. Another study found that ZNHIT1, a major regulator of a chromatin remodeling complex, was necessary for heart function [
86]. Specifically, the loss of this chromatin regulator caused rapid HF and dysregulated calcium signaling. It was determined that the ZNHIT1 regulated CASQ1, a major regulator of calcium signaling in the sarcoplasmic reticulum, by altering the histone 2A variant and therefore chromatin regulation [
86]. It was also found that BAF60c, a chromatin remodeling complex also called SMARCD3, regulates cardiomyocyte growth through MEF2 and myocardin gene expression [
87].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11020579