Both natural killer T (NKT) and natural killer (NK) cells are innate cytotoxic lymphoid cells that produce inflammatory cytokines and chemokines, and their role in the innate immune response to tumors and microorganisms has been investigated. Especially, emerging evidence has revealed their status and function in the tumor microenvironment (TME) of tumor cells. As a recent strategy in cancer immunotherapy, the mobilization or restoration of endogenous NKT or NK cells by novel vaccines or therapies has become a focus of research. Several new modalities based on the characteristics of NKT and NK cells, including artificial adjuvant vector cells, chimeric antigen receptor-expressing NK or NKT cell therapy, or their combination with immune checkpoint blockade have been developed. This research examines challenges and future directions for improving these therapies.
- NKT cell
- NK cell
- dendritic cells
- antitumor effect
- cancer immunotherapy
1. Introduction
2. Characterization of iNKT Cells and Anti-Cancer Immunotherapy
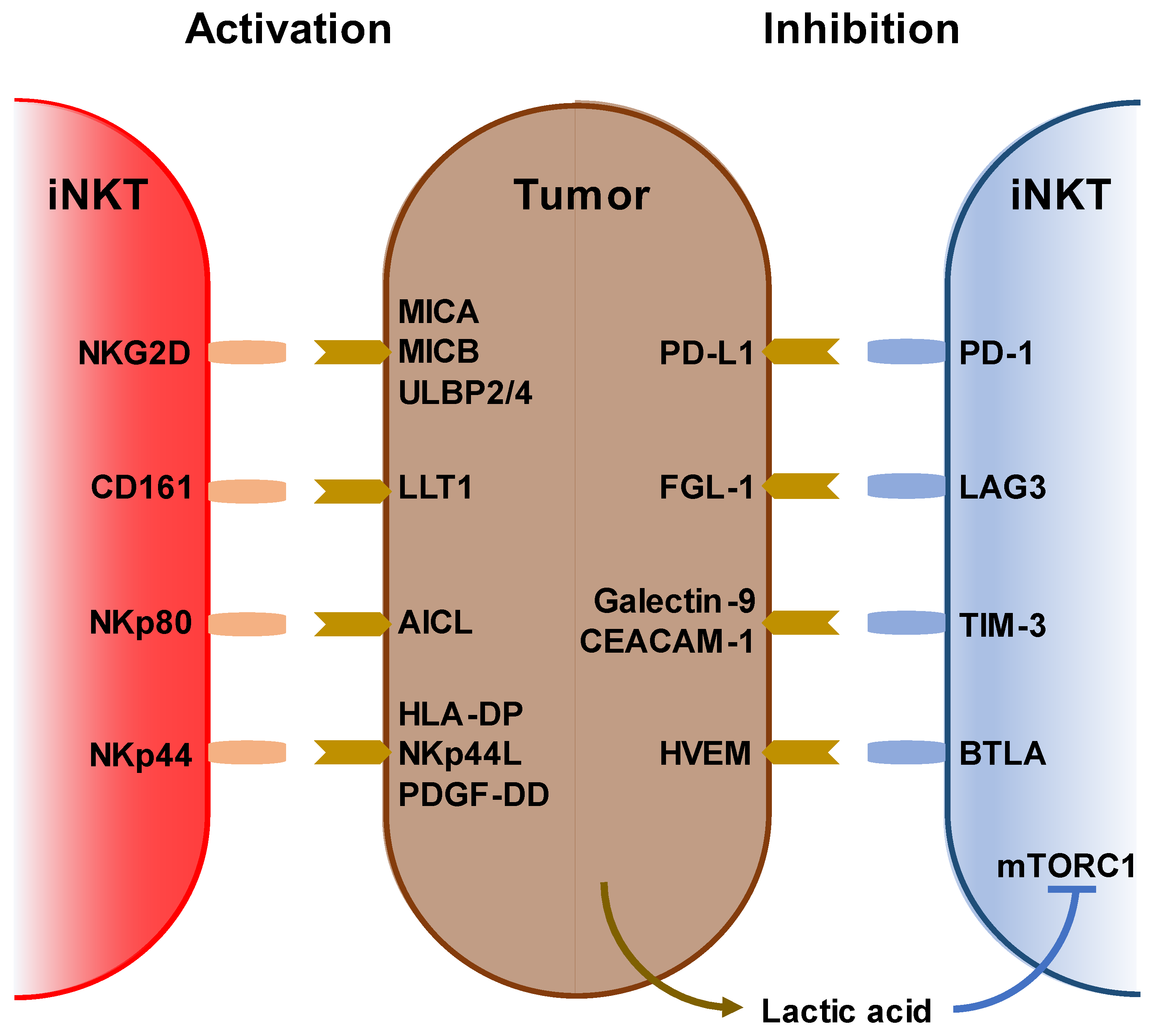
3. Immunological and Clinical Findings of iNKT-Based Immunotherapy
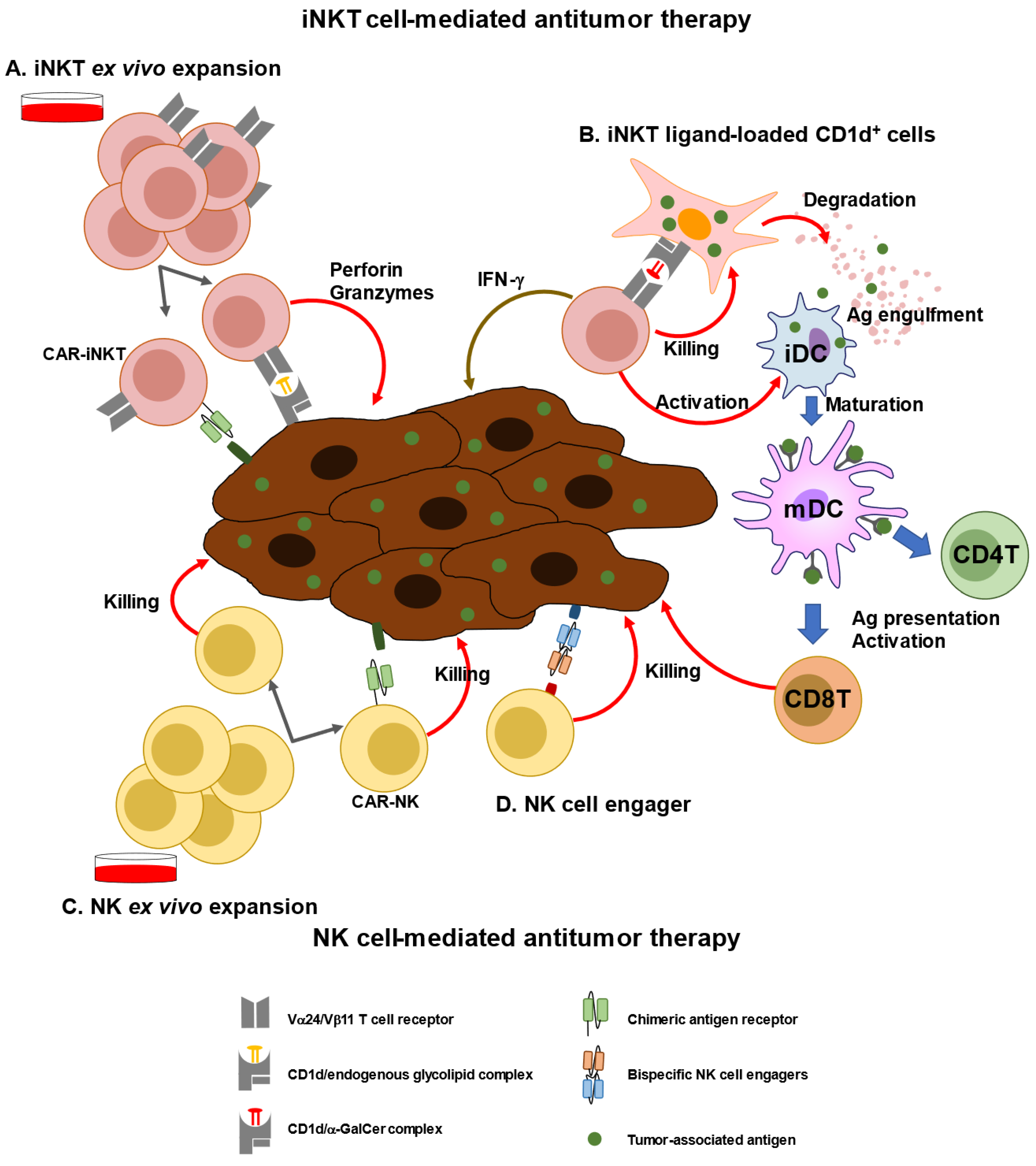
This entry is adapted from the peer-reviewed paper 10.3390/biom13020348
References
- Chevolet, I.; Speeckaert, R.; Schreuer, M.; Neyns, B.; Krysko, O.; Bachert, C.; Hennart, B.; Allorge, D.; van Geel, N.; Van Gele, M.; et al. Characterization of the in vivo immune network of IDO, tryptophan metabolism, PD-L1, and CTLA-4 in circulating immune cells in melanoma. Oncoimmunology 2015, 4, e982382.
- Marei, H.E.; Althani, A.; Caceci, T.; Arriga, R.; Sconocchia, T.; Ottaviani, A.; Lanzilli, G.; Roselli, M.; Caratelli, S.; Cenciarelli, C.; et al. Recent perspective on CAR and Fcgamma-CR T cell immunotherapy for cancers: Preclinical evidence versus clinical outcomes. Biochem. Pharmacol. 2019, 166, 335–346.
- Godfrey, D.I.; Le Nours, J.; Andrews, D.M.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473.
- Stolk, D.; van der Vliet, H.J.; de Gruijl, T.D.; van Kooyk, Y.; Exley, M.A. Positive & Negative Roles of Innate Effector Cells in Controlling Cancer Progression. Front. Immunol. 2018, 9, 1990.
- Fujii, S.; Shimizu, K. Immune Networks and Therapeutic Targeting of iNKT Cells in Cancer. Trends Immunol. 2019, 40, 984–997.
- Terabe, M.; Berzofsky, J.A. Tissue-Specific Roles of NKT Cells in Tumor Immunity. Front. Immunol. 2018, 9, 1838.
- Kato, S.; Berzofsky, J.A.; Terabe, M. Possible Therapeutic Application of Targeting Type II Natural Killer T Cell-Mediated Suppression of Tumor Immunity. Front. Immunol. 2018, 9, 314.
- Koseki, H.; Imai, K.; Nakayama, F.; Sado, T.; Moriwaki, K.; Taniguchi, M. Homogenous junctional sequence of the V14+ T-cell antigen receptor alpha chain expanded in unprimed mice. Proc. Natl. Acad. Sci. USA 1990, 87, 5248–5252.
- Lantz, O.; Bendelac, A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 1994, 180, 1097–1106.
- Fujii, S.; Shimizu, K.; Hemmi, H.; Steinman, R.M. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol. Rev. 2007, 220, 183–198.
- Porcelli, S.; Yockey, C.E.; Brenner, M.B.; Balk, S.P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J. Exp. Med. 1993, 178, 1–16.
- Dellabona, P.; Padovan, E.; Casorati, G.; Brockhaus, M.; Lanzavecchia, A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J. Exp. Med. 1994, 180, 1171–1176.
- Rogers, P.R.; Matsumoto, A.; Naidenko, O.; Kronenberg, M.; Mikayama, T.; Kato, S. Expansion of human Vα24+ NKT cells by repeated stimulation with KRN7000. J. Immunol. Meth. 2004, 285, 197–214.
- Coquet, J.M.; Chakravarti, S.; Kyparissoudis, K.; McNab, F.W.; Pitt, L.A.; McKenzie, B.S.; Berzins, S.P.; Smyth, M.J.; Godfrey, D.I. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4−NK1.1− NKT cell population. Proc. Natl. Acad. Sci. USA 2008, 105, 11287–11292.
- Crosby, C.M.; Kronenberg, M. Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 2018, 18, 559–574.
- Shissler, S.C.; Webb, T.J. The ins and outs of type I iNKT cell development. Mol. Immunol. 2019, 105, 116–130.
- Berzins, S.P.; Cochrane, A.D.; Pellicci, D.G.; Smyth, M.J.; Godfrey, D.I. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur. J. Immunol. 2005, 35, 1399–1407.
- Savage, A.K.; Constantinides, M.G.; Han, J.; Picard, D.; Martin, E.; Li, B.; Lantz, O.; Bendelac, A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 2008, 29, 391–403.
- Gumperz, J.E.; Miyake, S.; Yamamura, T.; Brenner, M.B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002, 195, 625–636.
- Lee, P.T.; Benlagha, K.; Teyton, L.; Bendelac, A. Distinct functional lineages of human valpha24 natural killer T cells. J. Exp. Med. 2002, 195, 637–641.
- Kuylenstierna, C.; Bjorkstrom, N.K.; Andersson, S.K.; Sahlstrom, P.; Bosnjak, L.; Paquin-Proulx, D.; Malmberg, K.J.; Ljunggren, H.G.; Moll, M.; Sandberg, J.K. NKG2D performs two functions in invariant NKT cells: Direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur. J. Immunol. 2011, 41, 1913–1923.
- Stojanovic, A.; Correia, M.P.; Cerwenka, A. The NKG2D/NKG2DL Axis in the Crosstalk Between Lymphoid and Myeloid Cells in Health and Disease. Front. Immunol. 2018, 9, 827.
- Winkler, I.; Wos, J.; Bojarska-Junak, A.; Semczuk, A.; Rechberger, T.; Baranowski, W.; Markut-Miotla, E.; Tabarkiewicz, J.; Wolinska, E.; Skrzypczak, M. An association of iNKT+/CD3+/CD161+ lymphocytes in ovarian cancer tissue with CA125 serum concentration. Immunobiology 2020, 225, 152010.
- Pisibon, C.; Ouertani, A.; Bertolotto, C.; Ballotti, R.; Cheli, Y. Immune Checkpoints in Cancers: From Signaling to the Clinic. Cancers 2021, 13, 4573.
- Li, Y.; Sharma, A.; Maciaczyk, J.; Schmidt-Wolf, I.G.H. Recent Development in NKT-Based Immunotherapy of Glioblastoma: From Bench to Bedside. Int. J. Mol. Sci. 2022, 23, 1311.
- Fu, S.; He, K.; Tian, C.; Sun, H.; Zhu, C.; Bai, S.; Liu, J.; Wu, Q.; Xie, D.; Yue, T.; et al. Impaired lipid biosynthesis hinders anti-tumor efficacy of intratumoral iNKT cells. Nat. Commun. 2020, 11, 438.
- Oh, S.F.; Jung, D.J.; Choi, E. Gut Microbiota-Derived Unconventional T Cell Ligands: Contribution to Host Immune Modulation. Immunohorizons 2022, 6, 476–487.
- Natori, T.; Morita, M.; Akimoto, K.; Koezuka, Y. Agelasphins, novel antitumor and immunostimulatory cerebrosides from the sponge Agelas mauritanus. Tetrahedron 1994, 50, 2771–2784.
- Ustjanzew, A.; Sencio, V.; Trottein, F.; Faber, J.; Sandhoff, R.; Paret, C. Interaction between Bacteria and the Immune System for Cancer Immunotherapy: The alpha-GalCer Alliance. Int. J. Mol. Sci. 2022, 23, 5896.
- Dias, B.R.; Rodrigues, E.G.; Nimrichter, L.; Nakayasu, E.S.; Almeida, I.C.; Travassos, L.R. Identification of iGb3 and iGb4 in melanoma B16F10-Nex2 cells and the iNKT cell-mediated antitumor effect of dendritic cells primed with iGb3. Mol. Cancer 2009, 8, 116.
- Cameron, G.; Cheng, J.M.H.; Godfrey, D.I.; Timmer, M.S.M.; Stocker, B.L.; Dangerfield, E.M. The NKT cell TCR repertoire can accommodate structural modifications to the lipid and orientation of the terminal carbohydrate of iGb3. RSC Adv. 2022, 12, 18493–18500.
- Wu, D.Y.; Segal, N.H.; Sidobre, S.; Kronenberg, M.; Chapman, P.B. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 2003, 198, 173–181.
- Tsuji, M. Glycolipids and phospholipids as natural CD1d-binding NKT cell ligands. Cell. Mol. Life Sci. 2006, 63, 1889–1898.
- Kain, L.; Costanzo, A.; Webb, B.; Holt, M.; Bendelac, A.; Savage, P.B.; Teyton, L. Endogenous ligands of natural killer T cells are alpha-linked glycosylceramides. Mol. Immunol. 2015, 68, 94–97.
- Tiwary, S.; Berzofsky, J.A.; Terabe, M. Altered Lipid Tumor Environment and Its Potential Effects on NKT Cell Function in Tumor Immunity. Front. Immunol. 2019, 10, 2187.
- Cox, D.; Fox, L.; Tian, R.; Bardet, W.; Skaley, M.; Mojsilovic, D.; Gumperz, J.; Hildebrand, W. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE 2009, 4, e5325.
- Lee, M.S.; Sun, W.; Webb, T.J. Sphingosine Kinase Blockade Leads to Increased Natural Killer T Cell Responses to Mantle Cell Lymphoma. Cells 2020, 9, 1030.
- Jahnke, S.; Schmid, H.; Secker, K.A.; Einhaus, J.; Duerr-Stoerzer, S.; Keppeler, H.; Schober-Melms, I.; Baur, R.; Schumm, M.; Handgretinger, R.; et al. Invariant NKT Cells From Donor Lymphocyte Infusions (DLI-iNKTs) Promote ex vivo Lysis of Leukemic Blasts in a CD1d-Dependent Manner. Front. Immunol. 2019, 10, 1542.
- Gorini, F.; Azzimonti, L.; Delfanti, G.; Scarfo, L.; Scielzo, C.; Bertilaccio, M.T.; Ranghetti, P.; Gulino, A.; Doglioni, C.; Di Napoli, A.; et al. Invariant NKT cells contribute to chronic lymphocytic leukemia surveillance and prognosis. Blood 2017, 129, 3440–3451.
- Li, Z.; Yang, B.; Zhang, Y.; Ma, J.; Chen, X.; Lao, S.; Li, B.; Wu, C. Mycobacterium tuberculosis-specific memory NKT cells in patients with tuberculous pleurisy. J. Clin. Immunol. 2014, 34, 979–990.
- Shimizu, K.; Sato, Y.; Shinga, J.; Watanabe, T.; Endo, T.; Asakura, M.; Yamasaki, S.; Kawahara, K.; Kinjo, Y.; Kitamura, H.; et al. KLRG+ invariant natural killer T cells are long-lived effectors. Proc. Natl. Acad. Sci. USA 2014, 111, 12474–12479.
- Shimizu, K.; Sato, Y.; Kawamura, M.; Nakazato, H.; Watanabe, T.; Ohara, O.; Fujii, S. Eomes transcription factor is required for the development and differentiation of invariant NKT cells. Commun. Biol. 2019, 2, 150.
- Prasit, K.K.; Ferrer-Font, L.; Burn, O.K.; Anderson, R.J.; Compton, B.J.; Schmidt, A.J.; Mayer, J.U.; Chen, C.J.; Dasyam, N.; Ritchie, D.S.; et al. Intratumoural administration of an NKT cell agonist with CpG promotes NKT cell infiltration associated with an enhanced antitumour response and abscopal effect. Oncoimmunology 2022, 11, 2081009.
- Fujii, S.; Shimizu, K. Exploiting Antitumor Immunotherapeutic Novel Strategies by Deciphering the Cross Talk between Invariant NKT Cells and Dendritic Cells. Front. Immunol. 2017, 8, 886.
- Krijgsman, D.; Hokland, M.; Kuppen, P.J.K. The Role of Natural Killer T Cells in Cancer-A Phenotypical and Functional Approach. Front. Immunol. 2018, 9, 367.
- Cortesi, F.; Delfanti, G.; Grilli, A.; Calcinotto, A.; Gorini, F.; Pucci, F.; Luciano, R.; Grioni, M.; Recchia, A.; Benigni, F.; et al. Bimodal CD40/Fas-Dependent Crosstalk between iNKT Cells and Tumor-Associated Macrophages Impairs Prostate Cancer Progression. Cell Rep. 2018, 22, 3006–3020.
- Mussai, F.; De Santo, C.; Cerundolo, V. Interaction between invariant NKT cells and myeloid-derived suppressor cells in cancer patients: Evidence and therapeutic opportunities. J. Immunother. 2012, 35, 449–459.
- Lam, P.Y.; Nissen, M.D.; Mattarollo, S.R. Invariant Natural Killer T Cells in Immune Regulation of Blood Cancers: Harnessing Their Potential in Immunotherapies. Front. Immunol. 2017, 8, 1355.
- Tahir, S.M.; Cheng, O.; Shaulov, A.; Koezuka, Y.; Bubley, G.J.; Wilson, S.B.; Balk, S.P.; Exley, M.A. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J. Immunol. 2001, 167, 4046–4050.
- Yanagisawa, K.; Seino, K.; Ishikawa, Y.; Nozue, M.; Todoroki, T.; Fukao, K. Impaired Proliferative Response of Vα24 NKT Cells from Cancer Patients Against α-galactosylceramide. J. Immunol. 2002, 168, 6494–6499.
- Dhodapkar, M.V.; Geller, M.D.; Chang, D.H.; Shimizu, K.; Fujii, S.; Dhodapkar, K.M.; Krasovsky, J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 2003, 197, 1667–1676.
- Motohashi, S.; Okamoto, Y.; Yoshino, I.; Nakayama, T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin. Immunol. 2011, 140, 167–176.
- Wienke, J.; Dierselhuis, M.P.; Tytgat, G.A.M.; Kunkele, A.; Nierkens, S.; Molenaar, J.J. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur. J. Cancer 2021, 144, 123–150.
- Boeck, C.L.; Amberger, D.C.; Doraneh-Gard, F.; Sutanto, W.; Guenther, T.; Schmohl, J.; Schuster, F.; Salih, H.; Babor, F.; Borkhardt, A.; et al. Significance of Frequencies, Compositions, and/or Antileukemic Activity of (DC-stimulated) Invariant NKT, NK and CIK Cells on the Outcome of Patients With AML, ALL and CLL. J. Immunother. 2017, 40, 224–248.
- Nelson, A.; Lukacs, J.D.; Johnston, B. The Current Landscape of NKT Cell Immunotherapy and the Hills Ahead. Cancers 2021, 13, 5174.
- Metelitsa, L.S.; Wu, H.W.; Wang, H.; Yang, Y.; Warsi, Z.; Asgharzadeh, S.; Groshen, S.; Wilson, S.B.; Seeger, R.C. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J. Exp. Med. 2004, 199, 1213–1221.
- Liu, D.; Song, L.; Wei, J.; Courtney, A.N.; Gao, X.; Marinova, E.; Guo, L.; Heczey, A.; Asgharzadeh, S.; Kim, E.; et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J. Clin. Investig. 2012, 122, 2221–2233.
- Richter, J.; Neparidze, N.; Zhang, L.; Nair, S.; Monesmith, T.; Sundaram, R.; Miesowicz, F.; Dhodapkar, K.M.; Dhodapkar, M.V. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood 2013, 121, 423–430.
- Ishibashi, F.; Sakairi, Y.; Iwata, T.; Moriya, Y.; Mizobuchi, T.; Hoshino, H.; Yoshida, S.; Hanaoka, H.; Yoshino, I.; Motohashi, S. A phase I study of loco-regional immunotherapy by transbronchial injection of alpha-galactosylceramide-pulsed antigen presenting cells in patients with lung cancer. Clin. Immunol. 2020, 215, 108457.
- Fujii, S.; Goto, A.; Shimizu, K. Antigen mRNA-transfected, allogeneic fibroblasts loaded with NKT-cell ligand confer antitumor immunity. Blood 2009, 113, 4262–4272.
- Shimizu, K.; Mizuno, T.; Shinga, J.; Asakura, M.; Kakimi, K.; Ishii, Y.; Masuda, K.; Maeda, T.; Sugahara, H.; Sato, Y.; et al. Vaccination with antigen-transfected, NKT cell ligand-loaded, human cells elicits robust in situ immune responses by dendritic cells. Cancer Res. 2013, 73, 62–73.
- Shimizu, K.; Yamasaki, S.; Shinga, J.; Sato, Y.; Watanabe, T.; Ohara, O.; Kuzushima, K.; Yagita, H.; Komuro, Y.; Asakura, M.; et al. Systemic DC Activation Modulates the Tumor Microenvironment and Shapes the Long-Lived Tumor-Specific Memory Mediated by CD8+ T Cells. Cancer Res. 2016, 76, 3756–3766.
- Yamasaki, S.; Shimizu, K.; Kometani, K.; Sakurai, M.; Kawamura, M.; Fujii, S. In vivo dendritic cell targeting cellular vaccine induces CD4(+) Tfh cell-dependent antibody against influenza virus. Sci Rep. 2016, 6, 35173.
- Zhu, Y.; Smith, D.J.; Zhou, Y.; Li, Y.R.; Yu, J.; Lee, D.; Wang, Y.C.; Di Biase, S.; Wang, X.; Hardoy, C.; et al. Development of Hematopoietic Stem Cell-Engineered Invariant Natural Killer T Cell Therapy for Cancer. Cell Stem Cell 2019, 25, 542–557.e9.
- Li, Y.R.; Zhou, Y.; Kim, Y.J.; Zhu, Y.; Ma, F.; Yu, J.; Wang, Y.C.; Chen, X.; Li, Z.; Zeng, S.; et al. Development of allogeneic HSC-engineered iNKT cells for off-the-shelf cancer immunotherapy. Cell Rep. Med. 2021, 2, 100449.
- Li, Y.R.; Zeng, S.; Dunn, Z.S.; Zhou, Y.; Li, Z.; Yu, J.; Wang, Y.C.; Ku, J.; Cook, N.; Kramer, A.; et al. Off-the-shelf third-party HSC-engineered iNKT cells for ameliorating GvHD while preserving GvL effect in the treatment of blood cancers. iScience 2022, 25, 104859.
- Heczey, A.; Courtney, A.N.; Montalbano, A.; Robinson, S.; Liu, K.; Li, M.; Ghatwai, N.; Dakhova, O.; Liu, B.; Raveh-Sadka, T.; et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: An interim analysis. Nat. Med. 2020, 26, 1686–1690.
