2. Characterization of iNKT Cells and Anti-Cancer Immunotherapy
iNKT cells from mice express an invariant TCR, which is formed by the rearrangement of the Vα14 and Jα18 TCRα gene segments. iNKT cells are preferentially paired with Vβ chains, including Vβ8.2, Vβ7, or Vβ2 TCR β gene segments [
10,
11,
12]. In humans, iNKT cells express a rearranged Vα24-Jα18 TCRα-chain associated with a Vβ11 TCRβ-chain [
13,
14]. When iNKT cells are stimulated by a ligand, such as α-galactosylceramide (α-GalCer), they can produce large amounts of cytokines, such as IFN-γ, TNF-α, IL-2, and IL-4 [
15,
16]. Murine iNKT cells can be categorized into at least three distinct functional subsets, iNKT1, iNKT2, and iNKT17 cells, and this classification is regulated by the transcription factors T-bet, GATA3, and RORγ, respectively [
17,
18]. Each iNKT cell subset produces typical cytokines upon activation (IFN-γ, IL-4, or IL-17). A small population of iNKT17 cells is located in the lung and subcapsular regions of lymph nodes (LNs) [
17]. Human iNKT cells develop within the thymus in a PLZF-dependent manner, similar to murine iNKT cells [
19,
20]. However, subsets of human iNKT cells are not as fully defined as those of mouse iNKT cells. Among CD4
+ iNKT cells, CD8
+ iNKT cells, and double-negative (DN) iNKT cells, DN iNKT and CD8
+ iNKT cells are similar to mouse iNKT1 cells, with increased IFN-γ secretion and cytotoxic function when activated [
21,
22]. CD4
+ iNKT cells produce a relatively higher amount of Th2-type cytokines, such as IL-4 and IL-13, than other subsets [
21,
22]. Human iNKT cells, particularly CD4
- iNKT cells, predominantly express certain NK-related markers, such as 2B4, NKG2D, DNAM-1, CD94, and NKG2A [
23]. The cytotoxicity of human iNKT cells against target cells may be mediated by either TCR or natural cytotoxicity receptor-mediated signaling.
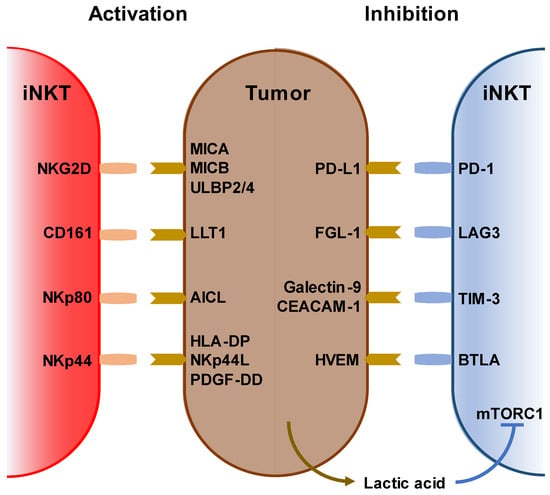
iNKT cells recognize and kill tumor cells via NKG2D, CD161, NKp44, and NKp80 as well as via endogenous NKT ligands loaded on the CD1d molecule. Conversely, tumor cells suppress iNKT cell function by expressing PD-L1, FGL-1, galectin-9, and HVEM [
24,
25,
26,
27]. Further, the tumor microenvironment is inhibitory to immune cells, and lactic acid inhibits IFN-γ production by blocking mTORC1 in iNKT cells and suppressing PPARγ-mediated cholesterol synthesis [
28] (
Figure 1).
Figure 1. Stimulatory and inhibitory receptors on iNKT cells. (Left) Ligands and receptors for activating iNKT cells. (right) Ligands and receptors for suppressing iNKT cells. Lack of oxygen increases lactic acid in the tumor microenvironment. Lactic acid also inhibits iNKT cell activation via mTORC1 signaling.
Owing to their peripheral differentiation, iNKT cells can differentiate into memory-type iNKT cells. iNKT cells recognize a variety of lipid antigens, including ceramide-based glycolipids, such as glycosphingolipids, microbial lipids, and endogenous self-lipids, presented by MHC class I-like CD1d molecules on antigen-presenting cells (APCs). These lipid antigens are either intermediates of APCs’ intracellular metabolism or originate from the cell walls of bacteria, fungi, or protozoan parasites.
Various α-linked ceramide glycolipids are antigenic glycolipids produced only by microorganisms. α-glucuronosylceramides and α-galacturonosylceramides are produced by
Sphingomonas spp., α-galactosyldiacylglycerols are produced by
Borrelia burgdorferi, and α-glucosyldiacylglycerols are produced by
Streptococcus pneumoniae [
29]. α-GalCer, a prototypical iNKT cell ligand, is a sphingolipid that was initially isolated from the marine sponge,
Agelas mauritiana, in 1994 [
30]. Although some human cells produce β-GalCer via the glycosphingolipid metabolic pathway, there is no direct pathway in human cells to produce α-GalCer. Recently, it was reported that α-GalCer, rather than β-GlcCer or β-GalCer, in mouse gut was affected by the presence of the antibiotic vancomycin and not colistin [
31]. Human gut bacteria, such as
Bacteroides fragilis,
Bacteroides vulgatus, and
Prevotella copri, produce α-GalCer structures that can activate iNKT cells. α-GalCer can also be detected in certain tumor tissues, such as colon adenocarcinoma tissues. In fact, the presence of
B. vulgatus in colon adenocarcinoma tissues correlates with better survival. Thus, α-GalCer-producing bacteria, members of the human gut microbiome, may infiltrate tumor tissues, resulting in antitumor activities [
31]. Endogenous CD1d-binding iNKT cell ligand glycolipids, such as isoglobotrihexosylceramide (iGb3) [
32,
33], disialoganglioside GD3 [
34], and α-glycosylceramides, have also been identified [
35,
36].
Tumor-derived lipids can help tumors evade immune surveillance using several mechanisms. An alteration in tumor lipid profile or the accumulation of specific lipids and fatty acids that favor tumor growth can limit antitumor immunity [
37]. Sphingosine-1-phosphate (S1P) expression is upregulated in mantle cell lymphoma (MCL), an aggressive subtype of non-Hodgkin lymphoma. Knockdown of sphingosine kinase 1, the enzyme responsible for producing S1P, in human MCL cells induces upregulation of the expression of cardiolipin and glycerophospholipid, which are abundant in mitochondrial membranes and bind to CD1d [
38], increasing iNKT cell activation [
39]. Another report showed that iNKT cells from the infusion of donor lymphocytes efficiently lysed leukemia cell lines and primary acute myeloid leukemia (AML) blasts in a dose- and CD1d-dependent manner [
40]. This implies that tumor metabolites present on tumor cell CD1d may be potential targets of iNKT-mediated immunotherapy against hematologic malignancies. Although CD1d expression varies among tumor types, in B cell chronic lymphocytic leukemia (CLL), the most common hematologic malignancy, the expression of CD1d is lower than that in normal B cells or is absent [
41], which might allow the tumor to escape from iNKT-mediated surveillance.
iNKT cells are part of the innate immune system, and effector memory-like iNKT cells have also been shown to function as other innate immune cells, such as NK and γδT cells, which differentiate into memory cells after activation. Human iNKT cells may mediate immune response against
Mycobacterium tuberculosis. In humans, CD3
+TCR Vβ11
+ NKT cells from pleural fluid mononuclear cells exhibit an effector memory phenotype. Increased levels of expression of cytolytic molecules and
M. tuberculosis antigens stimulate the production of IFN-γ, indicating that iNKT cells participate in local immune responses against
M. tuberculosis and protection against
M. tuberculosis infection [
42]. Killer cell lectin-like receptor subfamily G member 1-positive (KLRG1)-expressing iNKT cells were induced in the lungs after vaccination with α-GalCer-loaded CD1d
+ cells, including α-GalCer-loaded DCs or α-GalCer-loaded CD1d-expressing NIH-3T3 cells. KLRG1
+ iNKT cells co-expressing CD49d and granzyme A persisted for several months and retained a potent response to secondary stimulation [
43]. Shimizu et al. also revealed that Eomes plays two roles in iNKT cells. First, during iNKT cell development in the thymus, Eomes regulates iNKT1 cell differentiation. Second, Eomes promotes differentiation into effector memory cells in peripheral organs and spleen. Eomes-conditional deficient iNKT cells have been shown to fail to differentiate into KLRG1
+ granzyme A
+ long-term effector memory cells, even after stimulation with α-GalCer-loaded DCs [
44]. Furthermore, Prasit et al. reported that upon intratumoral administration of a TLR9 agonist, CpG and α-GalCer suppressed tumor growth in murine tumor models. This treatment also suppressed distant untreated tumors, known as the abscopal effect. They showed that sustained activity of iNKT cells was associated with the infiltration of KLRG1
+ effector memory iNKT cells into both tumors and regional LNs [
45].
3. Immunological and Clinical Findings of iNKT-Based Immunotherapy
Various therapies have been developed that are effective for treating hematopoietic tumors. Activating iNKT cells is an attractive strategy for anti-cancer therapy for several reasons. First, iNKT cells are available for treating many types of cancers because all human beings share the same invariant TCR and CD1d [
5,
46]. Second, iNKT cells can not only kill cancer cells directly but also induce activation of NK cells as an adjunct effect [
47]. Third, iNKT cells exert an adjuvant effect on T cells via DCs. Fourth, iNKT cells can alter the tumor microenvironment by inhibiting immunosuppressive cells, such as tumor-associated macrophages and myeloid-derived suppressor cells [
48,
49]. Although iNKT cell level is often decreased in patients with certain cancers, whether iNKT cells can be expanded or not is of higher importance than their level in patients [
50]. Reduced levels and functional impairment of iNKT cells have been reported in a wide range of solid and hematologic malignancies, including prostate [
51], lung, esophageal, colorectal, gastric, gallbladder, uterine, bile duct, and pancreatic cancers [
52] and multiple myeloma [
53] as well as in patients with late stage head and neck squamous cell carcinoma (HNSCC) [
54], neuroblastomas [
55], AML, and CLL [
56]. Conversely, increased level of intratumoral iNKT cells is correlated with good clinical outcomes and improved survival in colorectal cancer, neuroblastoma, periampullary adenocarcinoma, and hematologic malignancies [
57]. In prostate cancer and oral cell squamous carcinoma, iNKT cells exhibit defective IFN-γ production but acquire IL-4 production ability [
51]. Studies on cancer patients also showed that iNKT cells respond to chemotactic signals released by tumor cells, particularly in a range of primary and metastatic solid tumors. iNKT cell infiltration is associated with CCL2 expression in neuroblastoma cells [
58] and CCL20-producing tumor-associated macrophages [
59]. Patient-derived iNKT cells can be restored in vitro by culturing them in the presence of IL-2 or IL-12 or in vivo upon therapeutic administration of APCs pre-loaded with α-GalCer. Therefore, several clinical trials using autologous α-GalCer-pulsed DCs or adoptive transfer of ex vivo expanded iNKT cells have been conducted. α-GarCer-pulsed DC injections were well tolerated in all patients, with no major toxicity or altered immune response. The best clinical responses included decreased urine or serum M protein level in three cases of myeloma, one stable disease in a patient with renal cell cancer, and a reduction in tumor-associated monoclonal immunoglobulin level by administering α-GarCer-pulsed DC in combination with low-dose lenalidomide in three of four patients with measurable disease [
60]. In patients with advanced lung cancer, IFN-γ-producing iNKT cell therapy, including Vα24
+ iNKT cell and α-GalCer-pulsed APC (APC/Gal) therapy, correlated with clinical responses in 17 patients with non-small cell lung cancer [
61]. We established an mRNA-transfected cell-based vaccine (the artificial adjuvant vector cell (aAVC) system [
62,
63,
64]) that was co-transfected with an antigen-derived mRNA and CD1d mRNA, and subsequently loaded with iNKT cell ligand. When administered intravenously, aAVC activated iNKT and NK cells, and the activated iNKT and NK cells rejected aAVC. Subsequently, the killed aAVCs were taken up by DCs in situ, thereby activating several DC-specific immunogenic features. Previous studies on tumor therapeutic models reported that administration of tumor antigen (e.g., melanoma-associated antigen recognized by T cells-1 and Wilms’ tumor 1 (WT1))-expressing aAVCs induced potent CD4
+ and CD8
+ T cell responses [
63,
64,
65].
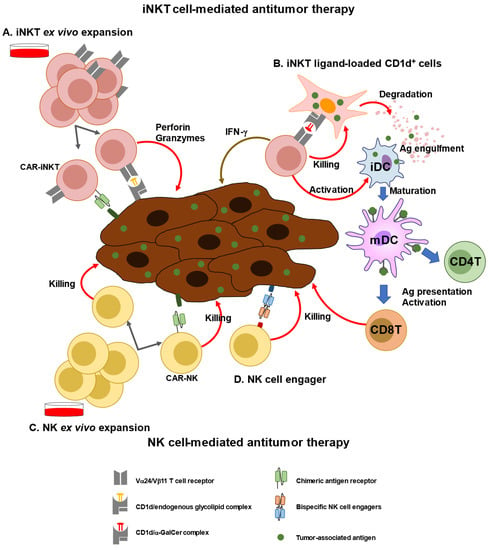
Next generation engineered iNKT cell transfer approaches have already been introduced (
Figure 2). Engineered human hematopoietic stem cells (HSCs) expressing a human iNKT TCR gene were examined for their ability to produce human iNKT cells that can target cancer. These HSC-derived iNKT cells effectively suppressed multiple myeloma cells in a xenograft model in a CD1d-dependent manner [
76]. Using genetically engineered CD34
+ HSCs, yield and purity of human allogeneic CD34
+ HSC-engineered iNKT (
AlloHSC-iNKT) cells have been increased to increase the number of endogenous iNKT cells.
AlloHSC-iNKT cells exhibited enhanced tumor-killing efficacy against five tumor cell lines due to increased expression of NK-activating receptors and increased production of highly cytotoxic molecules. BCMA-targeting CAR-engineered
AlloHSC-iNKT cells targeting MM tumors using the NK/TCR/CAR triple mechanism exhibited increased cell-killing activity [
77]. Furthermore, third-party HSC-iNKT cells are useful as preclinical models of lymphoma and leukemia [
78]. Moreover, engineered NKT cells co-expressing both anti-GD2 CAR and IL-15 were used in a phase I study for children with relapsed or resistant neuroblastoma. The results showed that CAR-NKT cells were expanded in vivo and migrated to the tumor site, resulting in the regression of bone metastatic lesions in one patient [
70].
Figure 2. iNKT or NK cell-mediated immunotherapy. (A) iNKT cells can be expanded ex vivo using specific ligands (i.e., a-GalCer) and cytokines (e.g., IL-2, and IL-15). Expanded iNKT cells or chimeric antigen receptor (CAR)-transfected NKT cells can be adoptively transferred to patients. (B) As a new modality of vaccines, iNKT ligand-loaded CD1d+ cells with tumor-associated antigen (TAA), called artificial advent vector cells, can induce TAA-specific CD4+ and CD8+T cells via maturation of dendritic cells. (C) NK cells can be expanded ex vivo using cytokines, such as IL-15 and IL-2. CAR-transfected NK cells can be adoptively transferred to patients. (D) Bispecific NK cells exhibit cytotoxic activity against tumor cells by promoting cell–cell interaction.