Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Microbiology
L-asparaginase (L-ASNase) is a vital enzyme with a broad range of applications in medicine, food industry, and diagnostics. Among various organisms expressing L-ASNases, thermophiles and hyperthermophiles produce enzymes with superior performances—stable and heat resistant thermo-ASNases.
- thermophiles
- L-asparaginase
- thermo-ASNase
- key metabolic enzyme
- thermostability
- structural features
1. Introduction
Thermophiles and hyperthermophiles are unique microorganisms that not only survive but also thrive at high temperatures. Among environmental factors temperature is unique in that it crosses physical barriers [1]. As a result, organisms cannot efficiently shield themselves from temperature in the way they can shield themselves from extreme external pH or salinity by maintaining steep concentration gradients over biological membranes. Each biomolecule inside microorganisms must be adapted to the temperatures in which they grow [1].
Thermophiles and hyperthermophiles are generally categorized based on the optimum growth temperatures. No consensus has been reached regarding the exact temperature range of each category [1][2][3]; here, the researchers use the following: thermophiles (TP) grow in the range of temperatures from 50 °C to 80 °C, whereas hyperthermophiles (HP) live in extremely hot environments with temperatures over 80 °C.
To thrive in such habitats, microorganisms use a combination of adaptive mechanisms. Studies of these specific mechanisms are carried out on each level of hierarchy, from microbial communities to cells and molecules. The area of special interest is elucidation of the molecular basis of thermophilic proteins’ stability and functionality under extreme conditions. Intrinsic resistance of proteins to high temperatures remains a fascinating puzzle of fundamental significance. Moreover, understanding the mechanisms underlying their thermostability is key for designing enzymes that can work in harsh conditions. Thus, studying the structural and functional features of the thermophilic/hyperthermophilic proteins as important basic components of metabolic pathways creates the basis to obtain highly active and stable forms of biotechnology relevant enzymes with broad prospects.
Among the most important industrial enzymes, L-asparaginase (L-ASNase) stands out. L-ASNase (EC 3.5.1.1; L-asparagine amidohydrolase) catalyzes the conversion of the amino acid L-asparagine (L-Asn) to L-aspartic acid and ammonia [4] and is used in the pharmaceutical, biosensor and food industries [5][6][7].
The enzyme is firstly known as an important anticancer agent used for treatment of acute lymphoblastic leukemia and other related blood cancers worldwide. The enzyme selectively hydrolyzes the extracellular L-Asn. The inability of susceptible tumor cells to synthesize their own L-Asn leads to death of lymphoblastic cells by apoptosis [8][9].
L-ASNase is used in the food industry to prevent the acrylamide formation in commercial fried foods [10]. By catalyzing the hydrolysis of L-Asn, L-ASNase prevents the reaction of reducing sugars with this amino acid to form carcinogenic acrylamide [11].
L-ASNase is applied in biosensors for monitoring the level of L-Asn both for diagnostic purposes and in the food industry [12][13].
Low stability of the current commercial L-ASNases from mesophiles restricts enzyme industrial application, particularly in the food industry, where temperatures shoot up to 120 °C or even beyond, resulting in a relatively rapid loss of their activity.
Relatively recently, thermophiles, and in particular hyperthermophiles, have been reported for their potential to produce L-ASNases with extraordinary properties. L-ASNases have been already characterized from archaea Thermococcus kodakaraensis [14][15][16], Thermococcus zilligii [17], Thermococcus gammatolerans [18], Thermococcus sibiricus [19], Pyrococcus yayanosii CH1 [20], Pyrococcus furiosus [21][22][23][24], Pyrococcus horikoshii [25], Pyrococcus abyssi [26], Archaeoglobus fulgidus [27]; Pyrobaculum calidifontis [28], living under extremely high temperatures. Due to superior performances for different areas of biotechnology [29], investigation of the characteristic features of thermophilic/hyperthermophilic L-ASNases (thermo-ASNases) is of particular interest.
On the other hand, these enzymes carry out a set of specific essential tasks in the thermophilic/hyperthermophilic cells.
2. Thermophilic L-ASNases as Members of a Large Family: Types Identified and Classification Issues
L-ASNases are an amazingly diverse family of enzymes [30]. These enzymes have been isolated from various organisms including bacteria, archaea, fungi, and eukarya [6][31][32][33].
According to the historical classification, L-ASNases are divided into three major groups depending on amino acid sequence, expressing organism, substrate specificity, structural and biophysical properties: bacterial-type I and II L-asparaginases, plant-type L-asparaginases, and Rhizobial-type L-asparaginases [31]. Despite widespread acceptance, this classification, based on the source of the first enzymes discovered, can be misleading. L-ASNases of the three canonical types, i.e., bacterial-type, plant-type and R. etli-type, are found throughout several kingdoms of life [34][35][36][37].
The preponderance of L-ASNases studied are found to be optimally active at, or near, mesophilic temperatures (approximately 30–40 °C) and belong to bacterial-type L-asparaginases [38]. Thermophilic/hyperthermophilic counterparts are not so extensively characterized. According to the classification originally used for mesophilic enzymes, three types of thermo-ASNases are currently reported: bacterial-type I, bacterial-type II, and plant-type L-asparaginases.
Cytosolic bacterial type I L-ASNases are involved in nitrogen metabolism and appear to be expressed constitutively [30][39]. In mesophiles, these enzymes exhibit low affinity towards L-Asn with KM values in the millimolar range [35].
Periplasmic bacterial type II L-ASNases appear to participate in carbon metabolism and their expression is tightly regulated by different factors [39]. Type II enzymes of mesophilic origin exhibit high specific activity towards L-Asn with micromolar KM.
Plant-type L-ASNases have dual L-asparaginase and isoaspartyl aminopeptidase activity [35]. These enzymes are located in the periplasmic space and hydrolyze the side-chain amide bond of L-Asn or its β-peptides [40].They are low-affinity proteins (millimolar KM) that belong to the superfamily of N-terminal nucleophile hydrolases [35][40][41].
According to the classification commonly used, most of the characterized enzymes from hyperthermophilic archaea Pyrococcus sp. and Thermococcus sp. belong to bacterial-type I L-ASNases [14][19][25]. The degree of identity of L-ASNases of the Thermococcus genera is about 60–80%, and for L-ASNases of Pyrococcus it is slightly lower (54–65%) (Figure 1) [19][33].
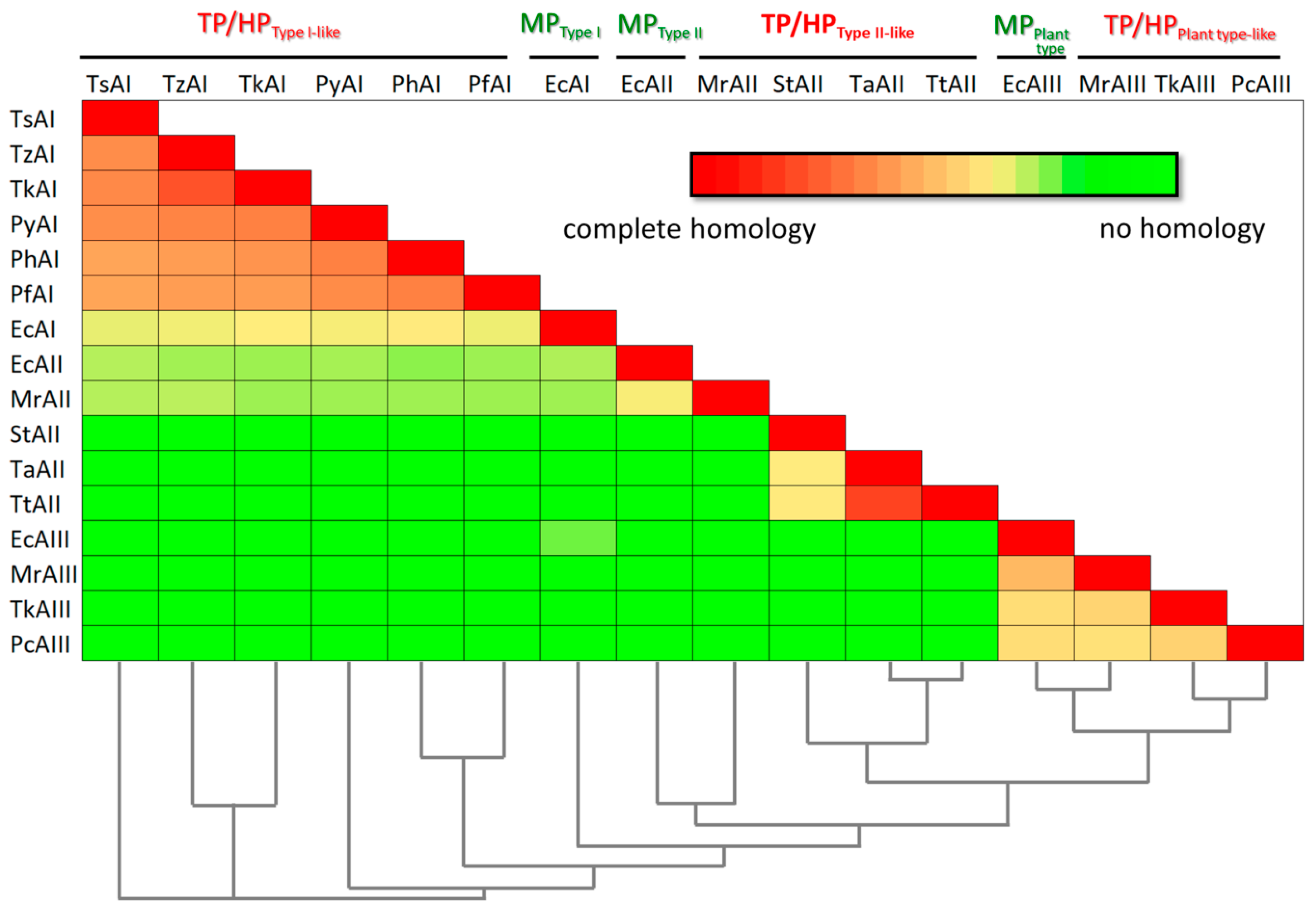
Figure 1. Amino acid identity matrix and phylogenetic relationships of mesophilic (MP), thermophilic (TP), and hyperthermophilic (HP) L-asparaginases from: TsAI—Thermococcus sibiricus (Accession Number: WP_015849943.1); TzAI—Thermococcus zilligii (Accession Number: WP_010478656.1); TkAI—Thermococcus kodakarensis (Accession Number: WP_011250607.1); PyAI—Pyrococcus yayanosii (Accession Number: WP_013906452.1); PhAI—Pyrococcus horikoshii (Accession Number: WP_010884185.1); PfAI—Pyrococcus furiosus (Accession Number: WP_011013191.1); EcAI—Escherichia coli (Accession Number: NP_416281.1); EcAII—Escherichia coli (Accession Number: AAA23445.1); MrAII—Melioribacter roseus (Accession Number: WP_014855710.1); StAII—Streptomyces thermoluteus (Accession Number: BAJ25701.1); TaAII—Thermus aquaticus (Accession Number: WP_003047338.1); TtAII—Thermus thermophilus (Accession Number: BDB12174.1); EcAIII—Escherichia coli (Accession Number: P37595.2); MrAIII—Melioribacter roseus (Accession Number: WP_014855981.1); TkAIII—Thermococcus kodakarensis (Accession Number: Q5JHT1.1); PcAIII—Pyrobaculum calidifonti (Accession Number: ABO08395.1).
At the same time, the sequence identity between hyperthermophilic bacterial-type I L-ASNases from archaea and type I L-ASNase from mesophilic bacteria Escherichia coli (EcAI, GenBank accession no. NP_416281.1, encoded by the ansA gene) used as a representative of this type [30][38] does not exceed 37% (Figure 1).
Most of the identified enzymes from thermophilic bacteria represent bacterial-type II L-ASNases. Examples include enzymes from Streptomyces thermoluteus subsp. fuscus NBRC 14270 (GenBank accession no. BAJ25701.1) [42], Melioribacter roseus (GenBank accession no. WP_014855710.1) [43], Thermus thermophilus (GenBank accession no. BDB12174.1), Thermus aquaticus (GenBank accession no. WP_003047338). Although being defined as type II L-ASNases, poor sequence identity between some of these enzymes is revealed: while the degree of identity between L-ASNases from Thermus sp. is more than 80%, the enzyme from Streptomyces thermoluteus subsp. fuscus NBRC shares only ~37% identity with L-ASNases from Thermus sp. No obvious sequence similarity (identity 10–12%) is found between these enzymes and L-ASNase from M. roseus. According to amino acid sequence alignment, type II L-ASNase from E. coli (EcAII, ansB, GenBank accession no. AAA23445.1), being a typical example of bacterial-type II ASNases, displays only 35.2% identity with M. roseus L-ASNase and 11–12% with counterparts from thermophilic bacteria mentioned above (Figure 1).
To date, the first three plant-type thermo-ASNases have been reported: from archaea Pyrobaculum calidifontis (ABO08395.1) and Thermococcus kodakarensis (Q5JHT1.1), and bacteria M. roseus (WP_014855981.1) [28][43][44]. Sequence identity in this group of thermo-ASNases does not exceed 39.5–43.8%. When compared with mesophilic plant-type L-ASNase from E. coli (EcAIII, ybiK, P37595), a similar degree of identity is observed—40.7–50.5% (Figure 1).
Although characterization of thermo-ASNases is far from complete, large variations in their properties are already evident, this being in keeping with the poor sequence identity between L-ASNases of TP/HP and mesophilic origin, as well as within the thermo-ASNase group.
In general, an increasing need for a revision of L-ASNases classification is noted in recent reviews [38][45]. While Schultz da Silva et al. have proposed to rename bacterial-type L-asparaginases, plant-type L-asparaginases, and Rhizobial-type L-asparaginases as Class 1, Class 2 and Class 3, respectively [45], Lubkowski and Wlodawer are the first who suggested separating HP archaeal L-ASNases in a distinct subgroup [38]. The authors proposed to group L-ASNases into three categories: tetrameric enzymes of type I and type II, and dimeric archaeal enzymes (HP L-ASNases) [38].
Unlike tetrameric mesophilic enzymes, L-ASNases expressed in HP archaea are homodimeric and sufficiently divergent. The enzymes display structural and topological properties that are distinct from either typical type I or type II L-ASNases, have active sites that deviate from the canonical arrangement seen in type I or II ASNases, or display kinetic/activity profiles that are at the intersection between type I and II ASNases [38].
Agreeing with the opinion of Lubkowski and Wlodawer, it should be noted that only the first characterized archaeal L-ASNases—Pyrococcus horikoshii (PhA), Pyrococcus furiosus (PfA), and Thermococcus kodakarensis (TkA)—were taken into consideration [38]. According to recent data, TP/HP microorganisms can possess several enzymes of different types exhibiting L-asparaginase activity [43][44]. For example, hyperthermophilic archaeon T. kodakarensis contains not only TkA more similar to members of the type I [14], but also a newly discovered TK2246 classified as a plant-type L-ASNase [44]. TK2246 displays low similarity with both TkA and typical plant-type L-ASNase E. coli EcAIII. In the pioneering research, homology modelling revealed structural changes between TK2246 and EcAIII—the presence of additional loop in TK2246 compared to EcAIII [44]. Moreover, gel filtration chromatography and SDS-PAGE indicated that TK2246 exists in a homodimeric form comprising two identical subunits [44]. At the same time, known mesophilic plant-type L-ASNases, including EcAIII, are heterotetramers or dimers of two αβ heterodimers [30][34]. In this view, it is likely that the subgroup of archaeal L-ASNases should be further divided into additional types.
The proposed classification of Lubkowski and Wlodawer concerns only the consideration of thermo-ASNases from archaea as a separate subgroup; nevertheless, thermo-ASNases of bacterial origin are also quite distinct from typical mesophilic L-ASNases. Also, there is no structural evidence, but it is supposed, that bacterial thermo-ASNases can act in oligomeric state different from di- or tetrameric, for example hexameric L-ASNase Thermus thermophilus (trimer of dimers) [30][46].
Initial classification criteria, such as comparison of amino acid homologies to EcAI and EcAII and/or substrate affinities (millimolar vs. micromolar, expressed in terms of KM) are also hardly applicable to thermophilic L-ASNases. For example, micromolar affinity to L-asparagine is defined as a specific trait of bacterial type II L-ASNases. Nevertheless, known type II L-ASNases from thermophilic bacteria show KM values in the millimolar range [6][43]. The level of substrate affinity in thermo-ASNases predetermined not by type, but rather the fact of adaptation of these organisms to high temperatures, far exceeding the appropriate for mesophiles. Increased KM values of various enzymes originating from thermophilic microorganisms compared to mesophilic counterparts confirm this conclusion: phosphoglycerate kinase [47], glutamate dehydrogenase [48], alkaline phosphatase [49][50][51], GTPase (TrmE) [52] and glucose-6-phosphate dehydrogenase [53]. In fact, successful attempts to improve the thermal stability of mesophilic L-ASNases have also resulted in a concomitant increase in KM [54]. It appears that adaptation at high environmental temperatures involves an increase in KM and kkat for thermo-ASNases [19]. This characteristic feature of thermo-ASNases allows optimizing catalytic efficiency by reaching a balance between substrate binding and the rate of product release.
In summary, there is a clear need to revise the current classification of L-ASNases. Considering the striking differences between mesophilic and TP/HP enzymes, probably associated with differences in the living environments, it must be recognized that thermo-ASNases must form a unique and novel group in the large family of L-ASNases and must be appropriately subdivided inside the group. This may be an extension of the classification proposed by Schultz da Silva et al., where thermo-ASNases would be classified into Class 4. Based on similarity in tertiary structures and general mechanisms within previously accepted types, and in an attempt to distinguish thermo-ASNases from mesophilic ones, Class 4 can be further subdivided into type I-like, type II-like, and plant-type-like (Figure 2).
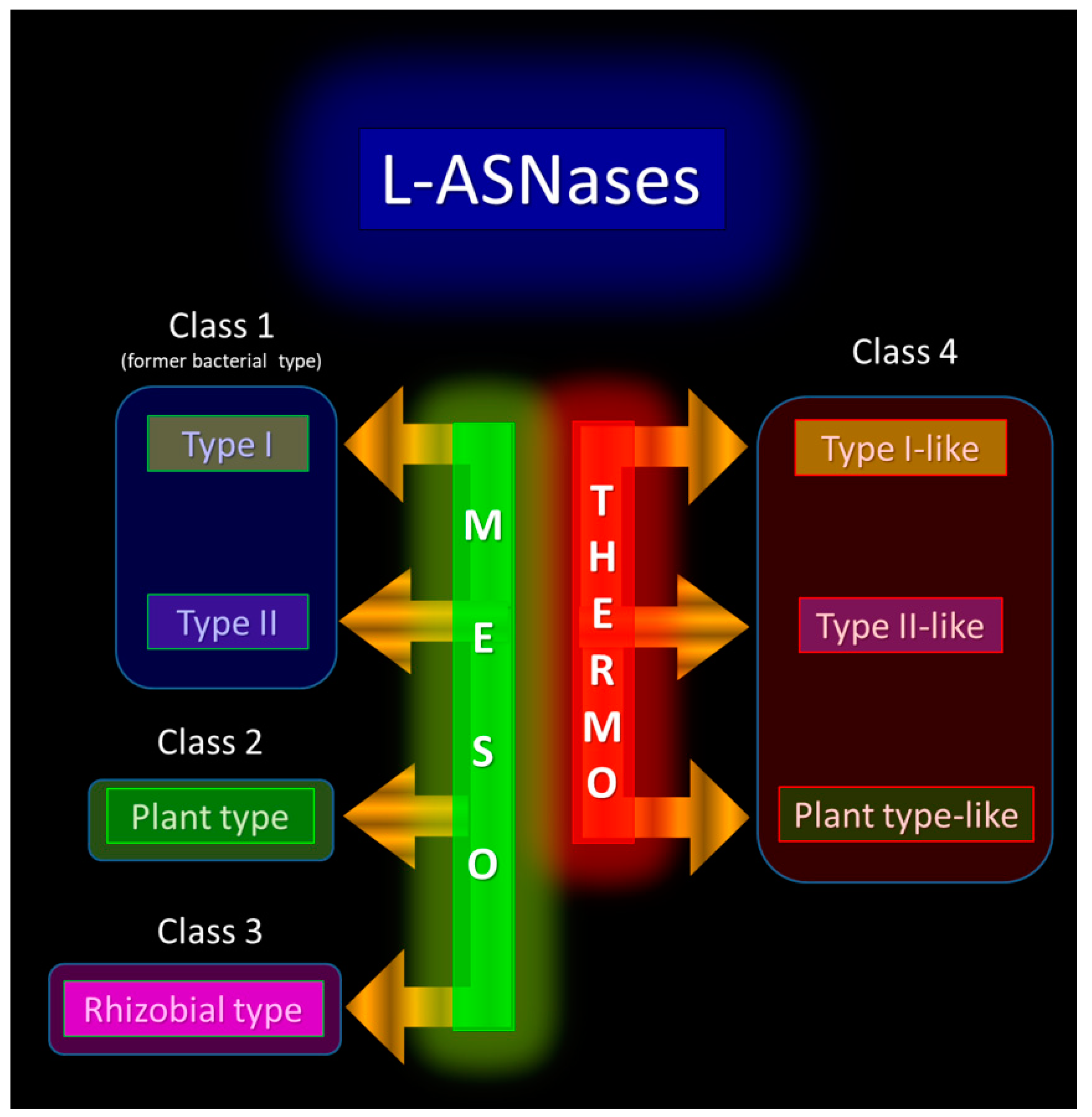
Figure 2. Proposed classification of L-ASNases, including thermo-ASNases as a novel Class 4.
3. L-ASNases as Key Metabolic Enzymes for Thermophilic and Hyperthermophilic Microorganisms
From the viewpoint of a living organism, L-ASNases catalyze a specific biochemical reaction that affects a number of metabolic processes, particularly those necessary for survival of TP/HP microorganisms at high temperatures (Figure 3).

Figure 3. Proposed scheme of multiple effects of thermo-ASNases on the survival of thermophilic (TP) and hyperthermophilic (HP) microorganisms under heat stress. Asp-derived—aspartate-derived, N—nitrogen, C—carbon, TCA—tricarboxylic acid cycle. (a) Aspartic acid, formed from asparagine by the action of thermo-ASNases, is associated with multiple metabolic pathways. (b) The role of thermo-ASNases in heat stress tolerance associated with the conversion of glutamine to glutamic acid: intensification of respiration and metabolism of amino acids. (c) Isoaspartyl aminopeptidase activity of thermo-ASNases as part of the protein repair system under heat-stress. (d) Maintenance of intracellular pH mediated by thermo-ASNases: thermo-ASNases by releasing ammonium ions can neutralize the excess proton influx under high temperatures. The diamond borders represent type I-like, type II-like and plant type-like thermo-ASNases. The red border is type I-like and type II-like thermo-ASNases associated with functions (a,b,d). The green border is plant type-like thermo-ASNases associated with functions (a,c,d).
L-ASNases catalyze the conversion of L-asparagine (L-Asn) to L-aspartic acid (or aspartate (Asp)) and ammonia. Asp acts as a critical metabolic hub to interconnect with diverse metabolic pathways (Figure 3a). According to recent data, Asp provides positive effects on mitigating abiotic stresses [55].
First of all, L-ASNases play a vital role in the biosynthesis of aspartate-derived amino acids, namely lysine, threonine, methionine and isoleucine, since aspartic acid is the precursor of these amino acids (Figure 3a) [56][57][58].
According to recent data, one of these amino acid—lysine—is involved in adaptation and tolerance to environmental stresses in various organisms [59][60]. Lysine is a charged amino acid; thus, its accumulation may contribute to the prevention of the protein denaturation caused by high-temperature stress. Due to the NH2 groups in the molecule, lysine functions as an ion-coating on the surface of membrane components and proteins to prevent denaturation. Furthermore, lysine is a kosmotropic compound due to the ammonium group in the side chain. The kosmotropic property of lysine may contribute to the protection of cells from the disordering of cellular macromolecules caused by high temperature, similar to the protective effect of compatible solutes against chaotropic stress [59]. As shown in a recent study, the increased level of lysine conferred high-temperature stress tolerance to E. coli cells [59].
Lei et al. have shown that Asp is able to enhance heat stress tolerance in plants by up-regulation of a total of nine amino acids—lysine, threonine, glutamate, asparagine, arginine, leucine, valine, glycine, and tryptophan [55].
Beyond its role as an amino acid in proteins, a precursor of amino acids and regulator of amino acid biosynthesis, aspartate is required for conversion of IMP to AMP in de novo purine synthesis and provides the carbon backbone for de novo pyrimidine synthesis (Figure 3a) [61]. The increased content of uracil, UMP, guanosine, and thymine by Asp under heat stress was reported for plants [55]. The authors conclude that Asp may contribute to the maintenance of RNA and DNA synthesis, supporting growth and defense against heat stress [55].
Thus, aspartate deficiency will impair protein, purine nucleotide, and pyrimidine nucleotide synthesis, resulting in decreased cell proliferation. Indeed, Sullivan et al. revealed that exogenous aspartate addition is sufficient to restore proliferation of cells that otherwise stop proliferating or die when activity of electron transport chain is impaired [61].
When excess amounts beyond the normal protein synthesis requirements are available, L-asparagine is hydrolyzed and utilized as a source of carbon and nitrogen [62]. L-ASNases of different types participate in nitrogen and carbon metabolism [35][39]. While expression of bacterial type II L-ASNases involved in carbon metabolism is tightly regulated by different factors [39], bacterial type I L-ASNases, engaged in nitrogen metabolism, appear to be expressed constitutively [30][39]. Asparagine is an important nitrogen storage and transport molecule, due to its relatively high nitrogen-to-carbon ratio (2:4, compared with 2:5 for glutamine, 1:5 for glutamic acid and 1:4 for aspartic acid, for example) and its relative chemical inertia. L-ASNases, participating in recycling the organically bound nitrogen through the ammonification process, play a notable role in nitrogen biogeochemical cycling [63].
In addition to asparaginase activity, some TP/HP L-ASNases have at least residual glutaminolytic activity [6]. Glutamate is known as an important component of several pathways, which links amino acid and respiration metabolism together (Figure 3b) [55]. Glutamate serves as a precursor of proline and arginine [55][64][65]. Arginine, in turn, is the biological precursor of nitric oxide (NO) and polyamines, which significantly contribute to stress tolerance [55][66][67][68]. TP/HP microorganisms require polyamines for growth at high temperatures [69]. Disruption of the genes involved in polyamine synthesis in the hyperthermophile T. kodakarensis resulted in severe growth defects at 85 °C, and even more so at 93 °C, which could be slightly reversed at 85 °C but not at 93 °C, by supplying exogenous polyamine spermidine [69].
T. thermophiles is also known to produce a variety of polyamines; the most common ones, spermidine and spermine, are synthesised using a distinct pathway from arginine via aminopropyl agmatine [1][66]. Polyamines of T. thermophilus were found to be necessary for the maintenance of the ribosome, tRNAHis, and tRNATyr structural integrity during growth at high temperatures [70].
In vitro biochemical studies indicate that polyamines induce structural changes to DNA that are proposed to facilitate growth at extreme temperatures [69][71].
In addition to glutaminase activity, Asp itself can increase accumulation of glutamate and arginine under heat stress. Asp-mediated activation of glutamate metabolism and the TCA enhances heat tolerance [55].
Thus, the direct metabolites of the biochemical reactions catalyzed by L-ASNases—Asp and Glu—are important nodes in the metabolic network of TP/HP microorganisms (Figure 3a,b).
Plant-type L-ASNases identified for TP/HP microorganisms [43][44] are known to have dual L-asparaginase and isoaspartyl aminopeptidase activity [35]. Isoaspartyl aminopeptidase activity protects plants from the spontaneous accumulation of highly toxic β-asparagine dipeptides (isoAsp) [35]. The role of plant-type L-ASNases in mesophilic microorganisms that also possess other types of the enzyme is not clear. Obviously, that is not the case with thermophiles and hyperthermophiles. The rate of isoAsp residue formation increases 910-fold at 90 °C compared with 23 °C [72]. Accumulation of proteins with altered aspartyl- and asparaginyl- residues is believed to be detrimental to cell survival at elevated temperatures [73]; hence, modified or damaged protein (peptide) should be rapidly digested to prevent cellular damage and to provide a potential nutrient source to support cell survival in harsh environments.
According to recent experimental data, heat shock-induced protein aggregates retarded growth of thermophilic bacteria, but expression of β-aspartyl peptidase (BAP) alleviated the growth defect by degrading damaged proteins [73]. BAP can directly hydrolyze isopeptide bonds, resulting in the release of Asp, and this might be an efficient repair mechanism for handing β-aspartyl-containing peptides [73].
Protein homeostasis, a balanced state between folded proteins and protein aggregates, is critical for cellular metabolism and physiology. In extremophiles thriving under harsh environments, in which proteins are vulnerable to protein inactivation and aggregation, cellular protein repair systems play a pivotal role in protein quality control to support cellular integrity and survival [73]. Spontaneous isopeptide bond formation, in particular, isoAsp residues, is accelerated at elevated temperatures [72][74], thus TP/HP L-ASNases with isoaspartyl aminopeptidase activity (plant-type L-ASNases) seems to act as a part of cellular protein repair system important for survival under extremely high temperatures (Figure 3c).
On the other hand, these enzymes may utilize β-aspartyl peptides as a source of amino acids to provide protein biosynthesis under stress conditions, for example, under nutrient depletion.
Another aspect of L-ASNase activity is releasing ammonium ions. Ammonia represents a direct metabolite of the biochemical reaction induced by the enzyme, and exerts profound effects on the electrochemical gradient, membrane potential, and the intracellular pH, thereby affecting the synthesis of ATP and activity of membrane transporters (Figure 3d) [75][76][77]. Singh et al. were the first who reported the influence of L-ASNase on intracellular pH-regulation and H+-gradient through ammonia releasing [76].
Ammonia (NH4+), the product of the enzymatic hydrolysis of Asn/Gln, could be involved in counteracting the H+-influx (acid stress) [76]. Indeed, it is known that the proton and sodium permeabilities of all biological membranes increase with the temperature [78]. The upper temperature border of life depends not only on the stability of the biomolecules, but, in addition, the proton permeability of membranes, which at higher temperatures may become too high to maintain electrochemical proton gradients in order to gain energy [79]. The increased motion of the lipid molecules in the membranes at high temperature causes an increased proton permeability, and in turn, the high proton leakage makes it impossible to control intracellular pH. Among TP/HP microorganisms, TP bacteria have more difficulties to restrict the proton permeation [78].
Thermo-ASNases by releasing ammonium ions can neutralize the excess proton influx, thus maintaining the much required intracellular environment (Figure 3d). The enzyme may be crucial in providing an immediate protective response in maintaining pH and aiding in survival at stress conditions [76]. It is interesting that E. coli exploits a similar survival mechanism to maintain an intracellular pH under environmental acidic stress: glutaminase converts Gln to glutamate (Glu) with concomitant release of ammonia, which neutralizes protons, resulting in elevated intracellular pH under acid stress [80].
Overall, these data confirm that thermo-ASNases are key metabolic enzymes, being a part of the complicated heat-tolerance cellular system in TP/HP microorganisms. All products of the enzymatic reaction are associated with multiple metabolic pathways. Playing crucial roles in the integration of cell metabolic systems, they contribute to survival under high temperature stressful conditions.
4. Toward a Molecular Understanding of Thermo-ASNases Thermostability
Thermo-ASNases are optimally active at temperatures close to, slightly below, or even above the optimal growth temperature of the host organisms. An analysis of the relationships between growth temperatures and optimal catalytic temperatures of enzymes revealed the influence of speciation rather than a common feature. Thus, archaeal thermo-ASNases from Pyrococcus sp. have optimum below the growth temperature. Another example is the thermo-ASNase from A. fulgidus, an enzyme that has an optimum of activity at 70 °C and the host strain has an optimum of growth at 83 °C.
According to previous data, many individual enzymes in TP and HP have catalytic optima much lower than the growth temperatures [1]. In addition, even the average activity of enzymes isolated from these organisms is 10–20 °C lower than the growth temperature. Extrinsic factors are proposed to provide enzyme adaptation with an optimum below the growth temperature in addition to adaptations in protein sequence and fold [1]. These factors may include compatible solutes such as diglycerol phosphate, found as a mechanism of protein thermostabilization in the hyperthermophiles, in particular, in archaea A. fulgidus [81].
Another character of the relationship between growth temperatures and enzyme optima can be observed for Thermococcus sp. These archaeal thermo-ASNases display an optimum higher than the growth temperature. It is likely that these enzymes themselves can serve as part of the first-line defense system under stress conditions, including heat stress. More active under elevated temperatures, they can quickly stabilize cellular systems providing an additional margin of safety for host cells.
Thermostability and optimal activity at high temperatures are inherent properties of thermo-ASNases. The differences in thermostability between TP/HP and mesophilic L-ASNases are striking. Previous studies reported that T. zilligii L-ASNase showed a slight decrease in activity after incubation for 2 h at 70–85 °C [17]. The half-life of T. kodakaraensis L-ASNase and T. gammatolerans L-ASNase at 85 °C was more than 120 min [15][82]. Storage of P. yayanosii L-ASNase at 37 °C for 1 month showed that 90% of the enzyme activity was retained [20]. The thermostability of non-thermophilic bacterial L-ASNases II is poor compared to thermo-ASNases. When incubated at 70 °C for 30 min, 80% of EcAII activity was lost. The half-life of EcAII at 50 °C (T(1/2, 50°C)) was estimated to be 60 min [54]. Mesophilic L-ASNase II from Bacillus subtilis retained approximately 14.7% of its activity after 2 h incubation at 50 °C and showed 9.0% residual activity after 2 h incubation at 60 °C [83].
Current studies agree that there is no unique feature or single mechanism responsible for the high functional activity at elevated temperatures and remarkable heat stability of TP/HP proteins [84][85][86]. A concerted action of structural, dynamic and other physicochemical attributes is utilized to ensure the delicate balance between stability and functionality of proteins at such harsh conditions.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24032674
References
- Engqvist, M.K.M. Correlating enzyme annotations with a large set of microbial growth temperatures reveals metabolic adaptations to growth at diverse temperatures. BMC Microbiol. 2018, 18, 177.
- Stetter, K.O. Hyperthermophilic Procaryotes. FEMS Microbiol. Rev. 1996, 18, 149–158.
- Imanaka, T. Molecular bases of thermophily in hyperthermophiles. Proc. Jpn. Acad. Ser. B 2011, 87, 587–602.
- Lopes, A.M.; de Oliveira-Nascimento, L.; Ribeiro, A.; Tairum, C.A.; Breyer, C.A.; de Oliveira, M.A.; Monteiro, G.; de Souza-Motta, C.M.; de Magalhães, P.O.; Avendaño, J.G.F.; et al. Therapeutic L-Asparaginase: Upstream, Downstream and Beyond. Crit. Rev. Biotechnol. 2017, 37, 82–99.
- Nunes, J.C.F.; Cristóvão, R.O.; Freire, M.G.; Santos-Ebinuma, V.C.; Faria, J.L.; Silva, C.G.; Tavares, A.P.M. Recent Strategies and Applications for L-Asparaginase Confinement. Molecules 2020, 25, 5827.
- Dumina, M.V.; Eldarov, M.A.; Zdanov, D.D.; Sokolov, N.N. L-Asparaginases of Extremophilic Microorganisms in Biomedicine. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2020, 14, 277–296.
- de Oliveira Lima, I.G.; Bispo, J.R.S.; da Silva, M.B.; de Oliveira Feitosa, A.; dos Santos, A.C.M.; Moreira, M.S.A.; Passarini, M.R.Z.; Câmara, P.E.A.S.; Rosa, L.H.; Oliveira, V.M.; et al. Technological Prospecting: Mapping Patents on L-asparaginases from Extremophilic Microorganisms. Recent Patents Biotechnol. 2021, 15, 250–265.
- Mahajan, R.V.; Kumar, V.; Rajendran, V.; Saran, S.; Ghosh, P.C.; Saxena, R.K. Purification and Characterization of a Novel and Robust L-Asparaginase Having Low-Glutaminase Activity from Bacillus licheniformis: In Vitro Evaluation of Anti-Cancerous Properties. PLoS ONE 2014, 9, e99037.
- Ali, U.; Naveed, M.; Ullah, A.; Ali, K.; Shah, S.A.; Fahad, S.; Mumtaz, A.S. L-asparaginase as a critical component to combat Acute Lymphoblastic Leukaemia (ALL): A novel approach to target ALL. Eur. J. Pharmacol. 2016, 771, 199–210.
- Muso-Cachumba, J.J.; Antunes, F.A.F.; Peres, G.F.D.; Brumano, L.; Santos, J.; Da Silva, S.S. Current applications and different approaches for microbial l-asparaginase production. Braz. J. Microbiol. 2016, 47, 77–85.
- National Toxicology Program. Report on Carcinogens, 14th ed.; Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, USA, 2019.
- Verma, N.; Kumar, K.; Kaur, G.; Anand, S.E. coli K-12 Asparaginase-Based Asparagine Biosensor for Leukemia. Artif. Cells Blood Substit. Biotechnol. 2007, 35, 449–456.
- Kumar, K.; Kataria, M.; Verma, N. Plant asparaginase-based asparagine biosensor for leukemia. Artif. Cells Nanomed. Biotechnol. 2012, 41, 184–188.
- Guo, J.; Coker, A.R.; Wood, S.P.; Cooper, J.B.; Chohan, S.M.; Rashid, N.; Akhtar, M. Structure and Function of the Thermostable L-Asparaginase from Thermococcus Kodakarensis. Acta Cryst. 2017, 73, 889–895.
- Chohan, S.M.; Rashid, N. TK1656, a thermostable l-asparaginase from Thermococcus kodakaraensis, exhibiting highest ever reported enzyme activity. J. Biosci. Bioeng. 2013, 116, 438–443.
- Hong, S.-J.; Lee, Y.-H.; Khan, A.R.; Ullah, I.; Lee, C.; Park, C.K.; Shin, J.-H. Cloning, expression, and characterization of thermophilicL-asparaginase from Thermococcus kodakarensis KOD1. J. Basic Microbiol. 2014, 54, 500–508.
- Zuo, S.; Zhang, T.; Jiang, B.; Mu, W. Reduction of acrylamide level through blanching with treatment by an extremely thermostable l-asparaginase during French fries processing. Extremophiles 2015, 19, 841–851.
- Zuo, S.; Xue, D.; Zhang, T.; Jiang, B.; Mu, W. Biochemical characterization of an extremely thermostable l-asparaginase from Thermococcus gammatolerans EJ3. J. Mol. Catal. B Enzym. 2014, 109, 122–129.
- Dumina, M.; Zhgun, A.; Pokrovskaya, M.; Aleksandrova, S.; Zhdanov, D.; Sokolov, N.; El’Darov, M. A Novel L-Asparaginase from Hyperthermophilic Archaeon Thermococcus sibiricus: Heterologous Expression and Characterization for Biotechnology Application. Int. J. Mol. Sci. 2021, 22, 9894.
- Li, X.; Zhang, X.; Xu, S.; Zhang, H.; Xu, M.; Yang, T.; Wang, L.; Qian, H.; Zhang, H.; Fang, H.; et al. Simultaneous cell disruption and semi-quantitative activity assays for high-throughput screening of thermostable L-asparaginases. Sci. Rep. 2018, 8, 7915.
- Bansal, S.; Srivastava, A.; Mukherjee, G.; Pandey, R.; Verma, A.K.; Mishra, P.; Kundu, B. Hyperthermophilic asparaginase mutants with enhanced substrate affinity and antineoplastic activity: Structural insights on their mechanism of action. FASEB J. 2012, 26, 1161–1171.
- Garg, D.K.; Kundu, B. Hyperthermophilic l -asparaginase bypasses monomeric intermediates during folding to retain cooperativity and avoid amyloid assembly. Arch. Biochem. Biophys. 2017, 622, 36–46.
- Garg, D.K.; Tomar, R.; Dhoke, R.R.; Srivastava, A.; Kundu, B. Domains of Pyrococcus furiosus l-asparaginase fold sequentially and assemble through strong intersubunit associative forces. Extremophiles 2015, 19, 681–691.
- Bansal, S.; Gnaneswari, D.; Mishra, P.; Kundu, B. Structural stability and functional analysis of L-asparaginase from Pyrococcus furiosus. Biochemistry 2010, 75, 375–381.
- Yao, M.; Yasutake, Y.; Morita, H.; Tanaka, I. Structure of the type IL-asparaginase from the hyperthermophilic archaeon Pyrococcus horikoshii at 2.16 Å resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2005, 61, 294–301.
- Nadeem, M.S.; Khan, J.A.; Al-Ghamdi, M.A.; Khan, M.I.; Zeyadi, M.A. Studies on the recombinant production and anticancer activity of thermostable L- asparaginase I from Pyrococcus abyssi. Braz. J. Biol. 2022, 82, e244735.
- Li, J.; Wang, J.; Bachas, L.G. Biosensor for Asparagine Using a Thermostable Recombinant Asparaginase from Archaeoglobus fulgidus. Anal. Chem. 2002, 74, 3336–3341.
- Chohan, S.M.; Rashid, N.; Sajed, M.; Imanaka, T. Pcal_0970: An extremely thermostable l-asparaginase from Pyrobaculum calidifontis with no detectable glutaminase activity. Folia Microbiol. 2018, 64, 313–320.
- Sajed, M.; Naeem, S.U.; Rashid, N. l-Asparaginases from hyperthermophilic archaea and their applications. In Microbial Extremozymes; Academic Press: Cambridge, MA, USA, 2021; pp. 177–184.
- Loch, J.I.; Jaskolski, M. Structural and biophysical aspects of L-asparaginases: A growing family with amazing diversity. Iucrj 2021, 8, 514–531.
- Jia, R.; Wan, X.; Geng, X.; Xue, D.; Xie, Z.; Chen, C. Microbial L-asparaginase for Application in Acrylamide Mitigation from Food: Current Research Status and Future Perspectives. Microorganisms 2021, 9, 1659.
- Bath De Morais, S.; de Souza, T.D.A.C.B. Human L-asparaginase: Acquiring knowledge of its activation (Review). Int. J. Oncol. 2021, 58, 11.
- Dumina, M.; Eldarov, M.; Zdanov, D.; Sokolov, N. L-asparaginases of extremophilic microorganisms in biomedicine. Biomeditsinskaya Khimiya 2020, 66, 105–123.
- Prahl, A.; Pazgier, M.; Hejazi, M.; Lockau, W.; Lubkowski, J. Structure of the isoaspartyl peptidase withL-asparaginase activity from Escherichia coli. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 1173–1176.
- Michalska, K.; Jaskolski, M. Structural aspects of L-asparaginases, their friends and relations. Acta Biochim. Pol. 2006, 53, 627–640.
- Nomme, J.; Su, Y.; Konrad, M.; Lavie, A. Structures of Apo and Product-Bound Human l-Asparaginase: Insights into the Mechanism of Autoproteolysis and Substrate Hydrolysis. Biochemistry 2012, 51, 6816–6826.
- Su, Y.; Karamitros, C.S.; Nomme, J.; McSorley, T.; Konrad, M.; Lavie, A. Free Glycine Accelerates the Autoproteolytic Activation of Human Asparaginase. Chem. Biol. 2013, 20, 533–540.
- Lubkowski, J.; Wlodawer, A. Structural and biochemical properties of L-asparaginase. FEBS J. 2021, 288, 4183–4209.
- Sharafi, Z.; Barati, M.; Khoshayand, M.; Adrangi, S. Screening for Type II L-Asparaginases: Lessons from the Genus Halomonas. Iran. J. Pharm. Res. 2017, 16, 1565–1573.
- Izadpanah Qeshmi, F.; Homaei, A.; Fernandes, P.; Javadpour, S. Marine microbial L-asparaginase: Biochemistry, molecular approaches and applications in tumor therapy and in food industry. Microbiol. Res. 2018, 208, 99–112.
- Bejger, M.; Imiolczyk, B.; Clavel, D.; Gilski, M.; Pajak, A.; Marsolais, F.; Jaskolski, M. Na+/K+ exchange switches the catalytic apparatus of potassium-dependent plantL-asparaginase. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 1854–1872.
- Hatanaka, T.; Usuki, H.; Arima, J.; Uesugi, Y.; Yamamoto, Y.; Kumagai, Y.; Yamasato, A.; Mukaihara, T. Extracellular Production and Characterization of Two Streptomyces l-Asparaginases. Appl. Biochem. Biotechnol. 2010, 163, 836–844.
- Dumina, M.; Zhgun, A.; Pokrovskaya, M.; Aleksandrova, S.; Zhdanov, D.; Sokolov, N.; El’Darov, M. Highly Active Thermophilic L-Asparaginase from Melioribacter roseus Represents a Novel Large Group of Type II Bacterial L-Asparaginases from Chlorobi-Ignavibacteriae-Bacteroidetes Clade. Int. J. Mol. Sci. 2021, 22, 13632.
- Chohan, S.M.; Sajed, M.; Naeem, S.U.; Rashid, N. Heterologous gene expression and characterization of TK2246, a highly active and thermostable plant type l-asparaginase from Thermococcus kodakarensis. Int. J. Biol. Macromol. 2020, 147, 131–137.
- da Silva, L.S.; Doonan, L.B.; Pessoa, A., Jr.; de Oliveira, M.A.; Long, P.F. Structural and functional diversity of asparaginases: Overview and recommendations for a revised nomenclature. Biotechnol. Appl. Biochem. 2021, 69, 503–513.
- Pritsa, A.A.; Kyriakidis, D.A. L-asparaginase of Thermus thermophilus: Purification, properties and identificaation of essential amino acids for its catalytic activity. Mol. Cell. Biochem. 2001, 216, 93–101.
- Bentahir, M.; Feller, G.; Aittaleb, M.; Lamotte-Brasseur, J.; Himri, T.; Chessa, J.-P.; Gerday, C. Structural, Kinetic, and Calorimetric Characterization of the Cold-active Phosphoglycerate Kinase from the Antarctic Pseudomonas sp. TACII18. J. Biol. Chem. 2000, 275, 11147–11153.
- Thomas, T.M.; Scopes, R.K. The Effects of Temperature on the Kinetics and Stability of Mesophilic and Thermophilic 3-Phosphoglycerate Kinases. Biochem. J. 1998, 330, 1087–1095.
- Copeland, W.H.; Nealon, D.A.; Rej, R. Effects of temperature on measurement of alkaline phosphatase activity. Clin. Chem. 1985, 31, 185–190.
- Abubakar, M.; Wasagu, R.; Umar, M. Kinetic Studies of Alkaline Phosphatase from the Liver of Agama agama Lizard. Niger. J. Basic Appl. Sci. 2013, 21, 122–126.
- Mahesh, M.; Neha, G.; Rajesh, T.S.; Somashekhar, R.; Puttaiah, E.T. Isolation and characterization of extracellular thermostable alkaline phosphatase enzyme from Bacillus spp. Int. J. Appl. Biol. Pharm. Technol. 2010, 1, 21–33.
- Singh, A.K.; Pindi, P.K.; Dube, S.; Sundareswaran, V.R.; Shivaji, S. Importance of trmE for Growth of the Psychrophile Pseudomonas syringae at Low Temperatures. Appl. Environ. Microbiol. 2009, 75, 4419–4426.
- Richer, H.B.; Brewer, J.; Fahlman, G.G.; Gibson, B.; Hansen, B.M.; Ibata, R.; Kalirai, J.S.; Limongi, M.; Rich, R.M.; Saviane, I.; et al. The Lower Main Sequence and Mass Function of the Globular Cluster Messier 4. Astrophys. J. 2002, 574, L151–L154.
- Li, X.; Zhang, X.; Xu, S.; Xu, M.; Yang, T.; Wang, L.; Zhang, H.; Fang, H.; Osire, T.; Rao, Z. Insight into the thermostability of thermophilic L-asparaginase and non-thermophilic L-asparaginase II through bioinformatics and structural analysis. Appl. Microbiol. Biotechnol. 2019, 103, 7055–7070.
- Lei, S.; Rossi, S.; Huang, B. Metabolic and Physiological Regulation of Aspartic Acid-Mediated Enhancement of Heat Stress Tolerance in Perennial Ryegrass. Plants 2022, 11, 199.
- Mesas, J.M.; Gil, J.A.; Martin, J.F. Characterization and Partial Purification of L-Asparaginase from Corynebacterium Glutamicum. J. Gen. Microbiol. 1990, 136, 515–519.
- Batool, T.; Makky, E.A.; Jalal, M.; Yusoff, M.M. A Comprehensive Review on l-Asparaginase and Its Applications. Appl. Biochem. Biotechnol. 2016, 178, 900–923.
- Azevedo, R.A.; Arruda, P.; Turner, W.L.; Lea, P.J. The biosynthesis and metabolism of the aspartate derived amino acids in higher plants. Phytochemistry 1997, 46, 395–419.
- Isogai, S.; Takagi, H. Enhancement of lysine biosynthesis confers high-temperature stress tolerance to Escherichia coli cells. Appl. Microbiol. Biotechnol. 2021, 105, 6899–6908.
- Kishor, P.B.K.; Suravajhala, R.; Rajasheker, G.; Marka, N.; Shridhar, K.K.; Dhulala, D.; Scinthia, K.P.; Divya, K.; Doma, M.; Edupuganti, S.; et al. Lysine, Lysine-Rich, Serine, and Serine-Rich Proteins: Link Between Metabolism, Development, and Abiotic Stress Tolerance and the Role of ncRNAs in Their Regulation. Front. Plant Sci. 2020, 11, 546213.
- Sullivan, L.B.; Gui, D.Y.; Hosios, A.M.; Bush, L.N.; Freinkman, E.; Vander Heiden, M.G. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell 2015, 162, 552–563.
- Schubert, C.; Zedler, S.; Strecker, A.; Unden, G. L-Aspartate as a high-quality nitrogen source in Escherichia coli: Regulation of L-aspartase by the nitrogen regulatory system and interaction of L-aspartase with GlnB. Mol. Microbiol. 2021, 115, 526–538.
- Sobat, M.; Asad, S.; Kabiri, M.; Mehrshad, M. Metagenomic discovery and functional validation of L-asparaginases with anti-leukemic effect from the Caspian Sea. Iscience 2021, 24, 101973.
- Van De Casteele, M.; Demarez, M.; Legrain, C.; Glansdorff, N.; Pierard, A. Pathways of arginine biosynthesis in extreme thermophilic archaeo- and eubacteria. J. Gen. Microbiol. 1990, 136, 1177–1183.
- Yoshida, A.; Tomita, T.; Atomi, H.; Kuzuyama, T.; Nishiyama, M. Lysine Biosynthesis of Thermococcus kodakarensis with the Capacity to Function as an Ornithine Biosynthetic System. J. Biol. Chem. 2016, 291, 21630–21643.
- Oshima, T. Unique polyamines produced by an extreme thermophile, Thermus thermophilus. Amino Acids 2007, 33, 367–372.
- Fukuda, W.; Morimoto, N.; Imanaka, T.; Fujiwara, S. Agmatine is essential for the cell growth of Thermococcus kodakaraensis. FEMS Microbiol. Lett. 2008, 287, 113–120.
- Morimoto, N.; Fukuda, W.; Nakajima, N.; Masuda, T.; Terui, Y.; Kanai, T.; Oshima, T.; Imanaka, T.; Fujiwara, S. Dual Biosynthesis Pathway for Longer-Chain Polyamines in the Hyperthermophilic Archaeon Thermococcus kodakarensis. J. Bacteriol. 2010, 192, 4991–5001.
- Michael, A.J. Polyamine function in archaea and bacteria. J. Biol. Chem. 2018, 293, 18693–18701.
- Nakashima, M.; Yamagami, R.; Tomikawa, C.; Ochi, Y.; Moriya, T.; Asahara, H.; Fourmy, D.; Yoshizawa, S.; Oshima, T.; Hori, H. Long and branched polyamines are required for maintenance of the ribosome, tRNAHis and tRNATyr in Thermus thermophilus cells at high temperatures. Genes Cells 2017, 22, 628–645.
- Nishio, T.; Yoshikawa, Y.; Fukuda, W.; Umezawa, N.; Higuchi, T.; Fujiwara, S.; Imanaka, T.; Yoshikawa, K. Branched-Chain Polyamine Found in Hyperthermophiles Induces Unique Temperature-Dependent Structural Changes in Genome-Size DNA. ChemPhysChem 2018, 19, 2299–2304.
- Ichikawa, J.K.; Clarke, S. A Highly Active Protein Repair Enzyme from an Extreme Thermophile: Thel-Isoaspartyl Methyltransferase fromThermotoga maritima. Arch. Biochem. Biophys. 1998, 358, 222–231.
- La, J.W.; Dhanasingh, I.; Jang, H.; Lee, S.H.; Lee, D.-W. Functional Characterization of Primordial Protein Repair Enzyme M38 Metallo-Peptidase From Fervidobacterium islandicum AW-1. Front. Mol. Biosci. 2020, 7, 600634.
- Si, M.; Xu, Q.; Jiang, L.; Huang, H. SpyTag/SpyCatcher Cyclization Enhances the Thermostability of Firefly Luciferase. PLoS ONE 2016, 11, e0162318.
- Jahns, T. Ammonium/urea-dependent generation of a proton electrochemical potential and synthesis of ATP in Bacillus pasteurii. J. Bacteriol. 1996, 178, 403–409.
- Singh, J.; Khan, M.I.; Yadav, S.P.; Srivastava, A.; Sinha, K.K.; Das, P.; Kundu, B. L-Asparaginase of Leishmania donovani: Metabolic target and its role in Amphotericin B resistance. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 337–349.
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956.
- Konings, W.N.; Albers, S.-V.; Koning, S.; Driessen, A.J.M. The cell membrane plays a crucial role in survival of bacteria and archaea in extreme environments. Int. J. Gen. Mol. Microbiol. 2002, 81, 61–72.
- Stetter, K.O. Extremophiles and their adaptation to hot environments. FEBS Lett. 1999, 452, 22–25.
- Lu, P.; Ma, D.; Chen, Y.; Guo, Y.; Chen, G.-Q.; Deng, H.; Shi, Y. L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res. 2013, 23, 635–644.
- Lamosa, P.; Burke, A.; Peist, R.; Huber, R.; Liu, M.-Y.; Silva, G.; Rodrigues-Pousada, C.; LeGall, J.; Maycock, C.; Santos, H. Thermostabilization of Proteins by Diglycerol Phosphate, a New Compatible Solute from the Hyperthermophile Archaeoglobus fulgidus. Appl. Environ. Microbiol. 2000, 66, 1974–1979.
- Shi, R.; Liu, Y.; Mu, Q.; Jiang, Z.; Yang, S. Biochemical characterization of a novel L-asparaginase from Paenibacillus barengoltzii being suitable for acrylamide reduction in potato chips and mooncakes. Int. J. Biol. Macromol. 2017, 96, 93–99.
- Jia, M.; Xu, M.; He, B.; Rao, Z. Cloning, Expression, and Characterization of l-Asparaginase from a Newly Isolated Bacillus subtilis B11–06. J. Agric. Food Chem. 2013, 61, 9428–9434.
- Unsworth, L.; Van Der Oost, J.; Koutsopoulos, S. Hyperthermophilic enzymes − stability, activity and implementation strategies for high temperature applications. FEBS J. 2007, 274, 4044–4056.
- Feng, C.; Ma, Z.; Yang, D.; Li, X.; Zhang, J.; Li, Y. A Method for Prediction of Thermophilic Protein Based on Reduced Amino Acids and Mixed Features. Front. Bioeng. Biotechnol. 2020, 8, 285.
- Vieille, C.; Zeikus, G.J. Hyperthermophilic Enzymes: Sources, Uses, and Molecular Mechanisms for Thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43.
This entry is offline, you can click here to edit this entry!
