Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Urbanization and industrialization are responsible for environmental contamination in the air, water, and soil. These activities also generate large amounts of heavy metal ions in the environment, and these contaminants cause various types of health issues in humans and other animals.
- heavy metals
- toxicity
- source of heavy metal ions
1. Introduction
Environmental contaminants are substances that are present in the natural environment at levels higher than their permissible limits [1]. Industrialization and urbanization have emerged as the major causes of environmental pollution over the last few decades. The utilization of natural resources at a careless rate creates disturbances in the environment and causes several related problems [2]. There are several types of pollutants, such as organic, inorganic, metallic, gaseous and biological pollutants, which contaminate the environment [3]. Contamination from metallic ions in water arises due to several natural and anthropogenic activities, which harm animals and plants [4].
Heavy metals are characterized by their higher atomic weight or higher density. The term ‘heavy metal’ is used to describe metalloids or metallic elements which have toxic effects on humans and other living organisms [5]. Heavy metals including arsenic (As), chromium (Cr), lead (Pb), and cadmium (Cd) are toxic to humans, but a few heavy metals are not toxic; these include gold (Au) [6]. The density of heavy metals is commonly greater than 5 g/cm3: for example, Cr, Cd, and Pb [7]. The human body is generally exposed to heavy metal ions in one of four ways: the ingestion of metal-contaminated food, drinking contaminated water, skin contact, and inhalation in metal-contaminated air [8]. Metal compounds tend to form covalent bonds, which are responsible for the extremely toxic nature of metalloid compounds. Heavy metals can become covalently attached to organic groups and form lipophilic compounds or ions [9]. Due to the lipophilic nature of these metallic compounds, they pass through the cell membrane and enter the cell. These metallic compounds cause toxic effects when they interact with cell organelles [10].
Various technologies are available for the minimization of toxic metal ions from water. Physiochemical methods, such as filtration, ion exchange, reverse osmosis, precipitation, and physical adsorption, are frequently used for heavy metal treatment [11]. Precipitation is a well-known technique for the removal of Cr (VI), as well as Pb (II) and Cd (II). The precipitation is undertaken by varying the pH of wastewater [12,13]. These physiochemical techniques are expensive and generate secondary chemical sludge; moreover, these methods are only effective when the concentration of heavy metals is higher in the water (above 2 mM) [14]. Considering the disadvantages of these physiochemical methods, there is an urgent need to develop cost-effective and eco-friendly methods for the successful removal of heavy metals from water [15].
2. The Source and Toxicity of Heavy Metal Ions
Several industrial processes, including leather tanning, chrome plating, battery manufacturing, the glass industries, agricultural activities, domestic waste, and pharmaceutical industrial processes, are considered major sources of heavy metals, which generate toxic metal ions in the environment [21,22]. According to the International Leadership Association, about ten million tons of Pb (II) have been generated; 85.10% of the total amount of Pb (II) produced has been used in the battery industry, 5.5% in pigments, and the rest in the miscellaneous category (2.1%) (ILA, 2017). Pb (II) is also used in petrol as tetraethyl and tetramethyl agents in the form of antiknocking compounds [23]. Cr (VI) is used for steel production, wood preservation, chrome plating, pigments, and electroplating [24,25]. Cd is generally used in the electroplating and battery industries [26]. The Central Pollution Control Board (CPCB) has demarcated the maximum discharge limit of heavy metals in industrial effluent (Table 1).
Table 1. Maximum dischargeable limits for heavy metals in industrial effluent [27].
| Heavy Metals | CPCB, 2000 (Dischargeable Limit in Industrial Effluent) |
|---|---|
| Cr (VI) | 1.00–2.00 mg/L |
| Cd (II) | 0.20–2.00 mg/L |
| Pb (II) | 0.10 mg/L |
The USA’s environmental protection agency (USEPA) has demarcated the maximum permissible limit of heavy metals in drinking water. The allowable concentrations of heavy metals in drinking water are listed in Table 2.
| Heavy Metals | US EPA, 2018 | WHO, 2011 |
|---|---|---|
| Cr (total) (mg/L) | 0.10 mg/L | 0.05 |
| Cd (II) (mg/L) | 0.005 mg/L | 0.003 |
| Pb (II) (mg/L) | 0.015 mg/L | 0.01 |
Beyond these permissible limits, heavy metals cause several toxic effects. Some heavy metals are considered potential carcinogens [30,31]. The toxicity of heavy metal ions is shown in Figure 1.
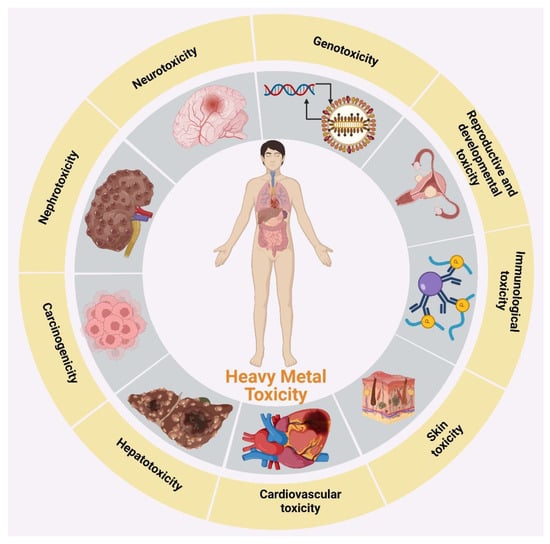
Figure 1. Toxicity of heavy metal ions.
Cr (VI) is a highly dangerous metallic ionic species, and its toxicity depends on its oxidation state [32,33]. It is a powerful oxidizing agent and shows much higher levels of toxicity than Cr (III). Cr (VI) enters the cell through the cell membrane and transforms into Cr (III) [34]. Cr (VI) reduces into Cr (III) and generates reactive oxygen species (ROS), which can damage internal components of the cell [35]. The Cr (VI) transformation process is known as a detoxification mechanism, which takes place away from the nucleus and other cell components. The reduction process also sometimes occurs outside of the cell through extracellular secretion [36]. Cr (VI)-reduction-mediated ROS generation can cause mutations in the DNA and damage cell organelles [37]. If the reduction of Cr (VI) occurs outside of the cell, Cr (III) is reduced and other intermediate reactions cannot deliver it into the cell, and toxicity is not observed [38]. Several researchers have noted that Cr (VI) is responsible for cancer and the damage of multiple organs, such as the liver and kidneys [39]. Gumbleton and Nicholls [40] indicated that Cr (VI) toxicity is responsible for kidney failure in rats. It has been reported that Cr (VI) is also responsible for respiratory cancer, the breakage of DNA strands, and abnormalities in chromosomes [41].
The main cause of Cd (II) exposure in humans is inhalation, through the ingestion of food and drinking of Cd (II)-contaminated water [42]. The chronic inhalation of Cd (II) causes a change in pulmonary functions, a reduction in olfactory functions, and emphysema [43]. Ingested Cd (II) causes abdominal pain, loss of consciousness, vomiting, nausea, hepatic injury, renal failure, gastrointestinal erosion, and a burning sensation [44]. It is also responsible for pulmonary adenocarcinomas and damage to single DNA strands, and disrupts the synthesis of proteins and nucleic acids [45].
Both natural and anthropogenic activities, such as mining, the burning of fossil fuels, and the manufacturing of batteries and glasses, are major sources of Pb (II) (https://fas.org/sgp/crs/misc/R46420.pdf (accessed on 16 June 2020). The toxic effects of Pb (II) in children come from dust and the chips used in packed food products, as it is used as a coating on the interior surfaces of packing materials [46]. The organs in the body that are most affected by Pb (II) toxicity are the kidneys, liver, and other soft tissues, such as the brain and heart [47]. Pb (II) toxicity massively impacts the nervous system. Poor attention, headaches, dullness, irritability, and memory loss are the early symptoms of Pb (II) poisoning in the central nervous system [48,49].
This entry is adapted from the peer-reviewed paper 10.3390/toxics11020147
This entry is offline, you can click here to edit this entry!
