Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dentistry, Oral Surgery & Medicine
Chronic atrophic candidiasis, commonly referred to as denture stomatitis (DS), is the most prevalent multifactorial, chronic inflammatory oral condition amongst denture wearers. It affects edentulous people who wear complete or partial dentures, as well as those who use intraoral removable orthodontic appliances and obturators. DS most commonly involves the palate and is more likely to be observed in patients with acrylic dentures than prostheses fabricated using other materials.
- antifungal drugs
- Candida albicans
- denture stomatitis
- oral candidiasis
1. Pathogenesis
The precise pathogenies of DS is not known, but mucosal trauma induced by ill-fitting dentures, sub-optimal oral hygiene, the nocturnal wearing of dentures, and xerostomia increase risk of infection with oral Candida [1,7]. The fitting surface of dentures provides a protected environment for the entrapment of yeast cells, which are able to colonize the irregularities in the denture-base and denture-relining materials [8]. This is more likely to occur in patients with other risk factors, such as poor oral hygiene and the continuous wearing of dentures.
Local risk factors associated with denture stomatitis are dry mouth, denture age, local trauma induced by an ill-fitting or poorly fabricated dentures, poor denture hygiene, microorganisms, continuous and nocturnal denture wearing, smoking, carbohydrate-rich diets, acidic salivary pH, and sensitivity to denture materials [7,9,10,11,12,13,14,15,16].
The oral microbiome of denture wearers is less diverse than those of fully dentate patients and may demonstrate higher rates of oral Candida carriage [17]. Candida displays dimorphism and can exist both in yeast and hyphal forms. The yeast form is observed in the carriage of oral Candida as commensals, while the hyphal form is associated with tissue invasion and disease, i.e., Candidiasis. Dentures provide a microenvironment that encourages Candida colonization [14]. The longstanding use of dentures in patients with poor oral hygiene allows the dental biofilm (plaque) to colonize the surface of the prosthesis and the mucosal surfaces in contact with the denture base [18,19].
Saliva has a dual role in Candidal adhesion to polymethylmethacrylate (PMMA). Saliva exhibits a physical cleansing effect and consists of antimicrobial components, such as lysozymes, immunoglobulins, glycoproteins, lactoferrin, and peroxidase. These constituents interact with Candida species and reduce their adherence and colonization on oral mucosal surfaces. However, some salivary proteins, such as mucins and statherins, can act as receptors for the nanoproteins present in Candidal cell walls and promote their adhesion [20]. Decreased salivary flow underneath the fitting surface of dentures further promotes the adhesion of Candida to the denture base and adjacent mucosal surfaces [21,22]. Ultimately, the Candida may develop into the hyphal form, infiltrate the mucosal tissue, and cause inflammation, which manifests clinically as DS.
The presence of a prosthesis is a prerequisite, and poor oral hygiene and the continuous use of dentures are the most significant risk factors for developing DS.
Oral mucosal trauma is also a risk factor for DS in susceptible patients and may result from ill-fitting or rocking full or partial dentures [23]. Both histological and microbiological analyses of mucosal tissue have shown that trauma has a significant role in the development of this condition [24]. Research suggests that trauma results in an inflammatory reaction, which creates a favorable environment for C. albicans to invade the tissues and initiate an inflammatory reaction [25]. Candidal growth has also been associated with the use of soft denture liners, often used to improve the fit of dentures. Soft liners are prone to deterioration, increasing their roughness and enhancing the risk of Candidal colonization [26]. Regarding prosthesis-related factors, an allergy in the form of contact mucositis may occur due to the presence of resin monomers, hydroquinone peroxide, dimethyl-p -toluidine, or methacrylate in the denture. Contact allergies are more common with cold- or auto-cured resins than with heat-cured denture-base materials due to the higher monomer content of the former [27]
Systemic risk factors include debilitating physiological aging, poorly controlled diabetes mellitus, nutritional deficiencies, immunosuppression, hematological disorders, and frequent use of broad spectrumantibiotics. These factors might contribute by compromising an individual’s resistance to combat the disease [7,9,14,15,21,22]. Recently, De Souza et al. [28] reported that individuals with low socioeconomic status are more likely to develop DS due to poor access to routine medical care. An association relationship between DS and other diseases, such as pneumonia and bacterial endocarditis, is also reported [3,19,28].
2. Classification, Prevalence, and Clinical Presentation
The first recognized classification of DS was proposed by Newton in 1962 [29], which still remains in use and groups DS as follows:
Type I: manifests as localized mucosal inflammation induced by trauma.
Type II: Diffuse involvement of the denture-bearing mucosa (Figure 1, Figure 2 and Figure 3).
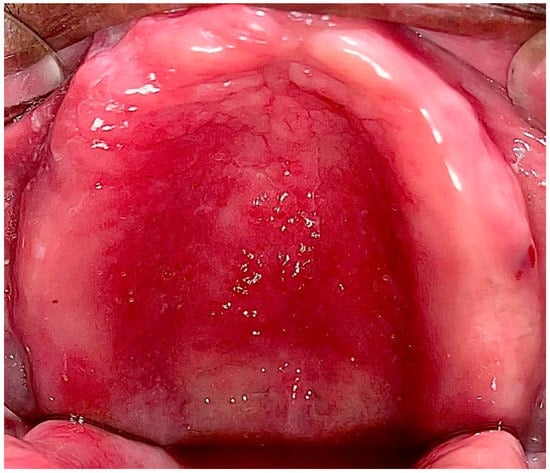
Figure 1. Diffuse involvement of the denture-bearing mucosa in a patient with an upper full denture.
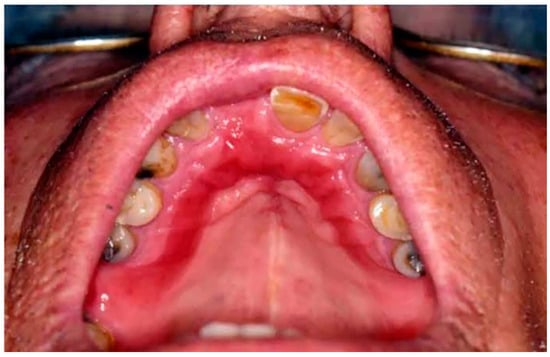
Figure 2. Diffuse involvement of the denture-bearing mucosa in a patient with an upper partial denture.
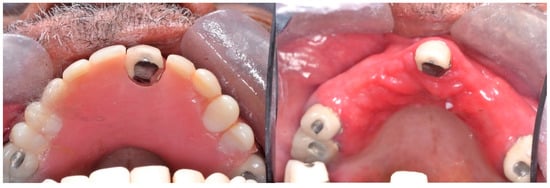
Figure 3. Diffuse involvement of the denture-bearing mucosa mirroring the partial denture base.
Type III: Additionally known as inflammatory papillary hyperplasia, the denture-bearing mucosa shows a granular appearance (Figure 4).
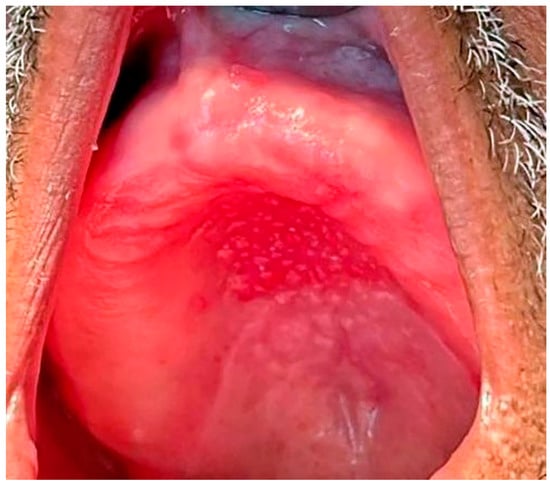
Figure 4. Inflammatory Papillary Hyperplasia in a patient with an upper full denture, the denture-bearing mucosa shows a granular appearance.
Although Newton’s classification is clinically useful for grading the hyperemia of denture-bearing mucosa, it does not accurately reflect the severity and extent of the disease [30].
A classification system has been proposed by Barbeau et al. [31] that subdivides Newton types into additional categories based on the extent of the lesions. The classification put forth by Schwartz et al. [32] subdivides Newton types I, II, and III based on the severity of inflammation using a score ranging from zero to six. Recently, Neppelenbroek et al. [30] proposed a modified Newton classification based on three clinical features: (a) appearance (I, II, and III), (b) the degree of inflammation of the palatal denture-bearing mucosa per quadrant, and (c) the degree of erythema (slight or intense redness). These scores for the three criteria are combined to rank the severity of DS using a total of 24 points.
One in every three denture wearers suffers from DS, the most common oral mucosal lesion associated with removable dentures [28]. The prevalence of DS ranges from 60 to 70% in denture wearers who exhibit clinical signs and symptoms [18,21]; however, this percentage may be up to 75% if asymptomatic patients are included [33].
The common sites of development of DS are the palate, tonsillar area, maxillary ridge, and posterior tongue [34,35]. In addition, Sartawi et al. [21] reported multiple small papillary nodules and papillary proliferations on the labial surface and labial mucosa, respectively. The conditions are rare in mandibular denture-bearing areas due to the washing action of saliva to clear the biofilm accumulation [36].
DS manifests clinically as variable mucosal erythema with or without dispersed petechiae in the areas covered by the denture base. The affected mucosa often has a palpable shape that mirrors the overlying denture. Despite the angry appearance of the affected mucosa due to marked erythema, most patients do not report any soreness. However, a minority of patients may report irritation of the affected oral mucosa, ulcerations, or a burning sensation, dysgeusia, dysphagia, and halitosis. Concurrent lesions commonly associated with DS include angular cheilitis, atrophic glossitis, and pseudomembranous or hyperplastic candidiasis [7,19,21]. The presence of prolonged inflammation may potentially be associated with cardiovascular disease, diabetes, pulmonary disease, and lead to the progression of systemic infections. [19,28].
This entry is adapted from the peer-reviewed paper 10.3390/ijerph20043029
This entry is offline, you can click here to edit this entry!
