Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Beer is one of the most consumed drinks worldwide. It contains numerous categories of antioxidants, phenolic products, traces of group B vitamins, minerals (selenium, silicon, potassium), soluble fibers and microorganisms. Low or moderate beer consumption, with or without alcohol, showed positive effects on health by stimulating the development of a healthy microbiota.
- beer
- microbiota
- polyphenols
- dietary fibers
1. Introduction
One of the most consumed drinks on the whole world is represented by beer. With a consumption history of several millennia, proven by very old archaeological discoveries, having a manufacturing technology that has evolved over time to the current form of the drink, it can have a significant impact on the health of people and the effects can be major, due to the enormous area where beer is drunk. In the past, beer also had medicinal values, as a stimulant or analgesic [1]. Beer was already widely consumed in ancient Egypt [2]. If until recently the history of beer went all the way to Babylonia, around 6000 BC, today there is evidence that a product similar to beer was consumed in China 9000 years ago, in a ritualic context [3]. Like other alcoholic beverages, beer is viewed with caution in the medical world. However, similar to other fermented products, it has health potential and beers without alcohol can be easily produced. It contains numerous categories of antioxidants, especially phenolic products from both hops and malt [4][5], traces of vitamins (especially from group B) [6] and minerals (selenium, silicon, potassium) [6] and soluble fibers [7]. Some bacteria and fungi have also been identified in the beer, the most encountered being lactic acid bacteria (Lactobacillales order, Bifidobacterium genus) and Saccharomyces spp. [8][9][10].
Alcohol itself can have dual effects, depending, of course, on the amount consumed. The composition, as well as the versatility of the manufacturing process that allows obtaining beer with minimal or no alcohol, identifies it as a potential functional food as such, but above all it can become functional through the addition of biologically active substances. Researchers exclude excessive consumption of alcoholic beer from the start, due to harmful effects. Numerous studies have followed the action of low or moderate beer consumption, with and without alcohol, on health. The identified effects were positive in the following directions: cardio-protective effect [11], bone health [12], positive stimulation of microbiota [13], etc. In the entire evaluation of beer, the current consumption trends must be taken into account, in which the individual is interested in the health benefits of the products consumed, beyond the satisfaction and covering of basic survival needs. The highlighting of the natural presence of some sanogenic ingredients or of the enrichment of the product in a specific ingredient with positive action on health can be beneficial for both the producer and the consumer [14].
In the human microbiota there are also Archaea and Viruses which roles are not yet completely understood [15]. The gut microbiota, composed of more than 100 trillion microorganisms [16], is the most studied, and several roles such as food fermentation, production of vitamins or even immune roles have been attributed to it. In humans, gut microbiota differs at the individual level, by localization into the gastro-intestinal tract and by age [17]. There are different conditions (pH and level of oxygen) that induce colonization with bacterial types: in the small intestine Proteobacteria (Enterobacteriaceae) are found and in the colon Bacteriodetes (Bacteroidaceae, Prevotellaceae and Rikenellaceae) were detected [18]. Gut microbiota becomes relatively stable from the age of three [19], but in people over seventy its diversity changes, with low levels of Bifidobacterium and high levels of Clostridium and Proteobacteria [20]. The alteration of healthy gut microbiota can determine an unbalanced composition of bacterial population, leading to various diseases such as cardiovascular diseases, cancer, diabetes mellitus, inflammatory bowel diseases, chronic liver diseases and chronic kidney diseases [21].
The relationship between beer or some of its ingredients and the intestinal microbiota is interesting and has been revealed in several studies to date, offering generous premises for future research. The influence of food ingredients on the composition, diversity and functionality of the intestinal microbiota is still incompletely deciphered, especially when it comes to lasting effects over time (Figure 1). It should be noted that chronic alcohol consumption has negative effects on the diversity of the microbiota, causing intestinal dysbiosis [13], among other ill effects on human metabolism. Researchers targeted four components of beer that might interact with gut microbiota, according to research: microorganisms, antioxidants, fiber and melanoidins. By means of pre and probiotic mechanisms, they show potential to enhance the development of a healthy gut microbiota, with predominant saccharolytic, short chain fatty acids-producing bacteria.

Figure 1. From beer to short chain fatty acids (SCFA).
2. Beer and its Principal Interactions with the Microbiota
2.1. The Microorganisms from Beer and Their Probiotic Potential
Beer is usually a pasteurized product, but there are crafted beers that have a potential of influencing the gut microbiota because they contain bacteria. Studies of the effect of beer consumption on the gut microbiota are few. A study from 2019 detected by sequencing of 16S rDNA eighteen genera of bacteria present in the rice beer, Lactobacillus being the dominant group (90%). Other types were Acetobacter, Acinetobacter, Bacillus, Dickeya, Enterococcus, Enterobacter, Exiguobacterium, Gluconobacter, Janibacteria, Klebsiella, Lactococcus, Leuconostoc, Pseudomonas, Pediococcus, Rothia, Staphylococcus and Weissella. Based on the detected bacteria and their bacterial profiles metabolic pathways were revealed as being influenced by the consumption of the rice beer, such as metabolisms of carbohydrate, amino acid, vitamins and cofactors, as well as xenobiotic biodegradation [22].
The main effect of alcohol intake on gut microbiota is dysbiosis [13][23], by changing the balance of the dominant bacterial from Phyla Bacteroidetes, Firmicutes and Phylum proteobacteria. However, this is not the case with usual beer, when consumed with moderation, because it contains about 5% alcohol. Low-alcohol and alcohol-free beers are popular and widely consumed. So, when considering beer, it is important to choose a type of beer with low or without alcohol, which gives the benefits of fermented foods.
Beer enriched with Saccharomyces cerevisiae strain intake may modulate gut microbiota and have beneficial influence the symptoms in Alzheimer’s disease, generating a neuroprotective effect by ameliorating cognition and increasing the concentration of anti-inflammatory cytokines, as new data revealed in 2022 [9]. Another type of beer rich in bacterial composition is Belgian lambic beer. The different bacteria present throughout the production process result because of the spontaneous inoculation of microorganisms from the environmental air and the inner surfaces of the wooden barrels [24]. Several new bacterial species such as Acetobacter lambici and Gluconobacter cerevisiae have been described in lambic beer. In the process of production of this type of beer, the following bacteria are present: Enterobacteria (Enterobacter cloacae; Klebsiella oxitoca), acetic acid bacteria (Acetobacter spp.; Gluconobacter cerevisiae) and lactic acid bacteria (Pediacoccus spp.), along with different yeasts (Hanseniaspora uvarum; Saccharomyces spp.; Brettanomyces spp.), with possible, not-yet-studied influence on gut microbiota. Crafted beers with several enhanced tastes, such as fruits, herbs, honey, spices and vegetables, have become more popular lately, but because they are not always pasteurized or sterilized by filtration they are subject to spoilage, due to the microbiota associated with the organic raw ingredients added to obtain the special tastes. Even if there is not enough knowledge available about the microbiota diversity in craft breweries it is known that some lactic acid bacteria from beer can produce biogenic amines such as histamine, tyrosine, putrescine and cadaverine, which can alter the beer and have possible toxic effects. Biogenic amines can also be found in sausages, fermented vegetables, fishery products, cheese and wine [25]. Published studies of sixty monitored points inside the craft brewery revealed that Lactobacillus, Pediococcus and Leuconostoc genera, are responsible for biogenic amines production, especially two isolates of Lactobacillus brevis that are able to be cultured into acidic conditions, with more hop and alcohol, these isolates had presented horA, horC and hitA genes, and the highest production of biogenic amines [26]. Corn beer has potential sources of probiotic lactic acid bacteria with cholesterol lowering activity, the strains identified by sequencing the 16S rRNA gene were Levilactobacillus brevis and Enterococcus faeccium, NCBI genbank accension numbers ON454506 and ON908682; isolates that effectively lowered LDL-c and increased HDL-c in rat sera, which are the main risk factors for cardiovascular diseases [27]. Beer is a fermented beverage that has enhanced nutritional and functional properties due to transformation of substrates, formation of bioactive end-products and presence of living microorganisms, genetically similar to strains used as probiotics [28]. Some studies revealed that beer components may have antimicrobial properties, as well as microbiological spoilage risks [29].
The microbial community (bacteria and fungi) from beer differs in time because it is influenced by its initial composition, the quantity of alcohol and the type of barrel where it is kept. Studies that used amplicon sequencing of the V4 region of the bacterial 16S rRNA gene and the fungal ITS1 region have shown using PerMANOVA analysis that during the process of beer maturation significant higher levels in the bacterial and fungal population appeared. The lactic acid bacteria became dominant in the moderately hopped beers and remained fairly constant in high-bitterness beer; with similar composition of the traditional beers, Pediococcus damnosus, Lactobacillus brevis and Acetobacter spp., and the fungi were influenced by the presence of alcohol [30]. There are even studies that introduced the idea of non-Saccharomyces yeast beers, and these types of beers may enter on the market in the future, after investigations and guideline for the safety assessment of yeasts are carried out [31].
So, bacteria and fungi encountered in the beer fabrication process, enriched or crafted beer, produce an array of compounds such as vitamins, bacteriocins and organic acids which confer health benefits to the consumers and can modulate the indigenous intestinal flora of the host.
2.2. Polyphenols and Microbiota
Beer is an important vehicle for polyphenols, which, together with bitter acids, form beer’s antioxidants. Most of them come from malt and only about 20% from hops. There are in vitro studies that confirm the action of polyphenols on the microbiota [32] (Figure 2). Animal studies support this interaction. For example, a polyphenol well represented in beer, ferulic acid, amplifies the biodiversity of the microbiota and stimulates the multiplication of bacteria that produce propionate and butyrate in the colon of rats [33]. Although limited in number, studies on human subjects also confirm the interaction under discussion. Studies show that after the ingestion of polyphenols, the production of short chain fatty acids (SCFA) increases, with consequent local anti-inflammatory effects [34]. The increase in SCFA synthesis confirms the action on the microbiota, an effect already demonstrated in the case of red wine consumption, which is itself a source of polyphenols. Red wine increased the levels of Bifidobacterium in the microbiota, as well as Faecalibacterium prausnitzii and Roeburia. The development of Enterobacter or Escherichia coli strains was inhibited [35]. These positive effects were also observed for alcohol-free wine. So, although the absorption of polyphenols from alcohol-free products (beer, wine) decreases compared to the same products with alcohol [4][36], polyphenols not absorbed that reach the colon have important effects in situ with multiple repercussions through the action on the intestinal microbiota. It is about oligo and polymeric polyphenols that usually do not undergo transformations until the distal intestine [34]. Polyphenols are “activated” by certain populations of the microbiota, especially when it comes to phytoestrogens [37][38][39]. Polyphenols are transformed by bacteria into absorbable products that reach through the portal blood, to the liver or into prenylated products that have important sanogenic actions, such as the antiproliferative action on some cell lines, as prenyl naringenin and xanthohumol have [40]. The interrelation between polyphenols and microbiota is extremely complex. The microbiota increases the bioavailability of polyphenols which, in turn, modulate the composition of the populations in the colon, inhibiting pathogenic microorganisms and stimulating the development of healthy ones through a prebiotic action [41][42]. Quercitin, a flavonoid from beer, combated intestinal dysbiosis, improving the ratio between Firmicutes and Bacteroides populations and opposing the proliferation of microbiota species associated with excessive body weight [43][44]. It should be noted that by drinking non-alcoholic beer or wine, you not only avoid the negative effects determined by ethyl alcohol, including on the microbiota, but also increase the number of polyphenols that reach the intestine and which would have positive effects on the microbiota.
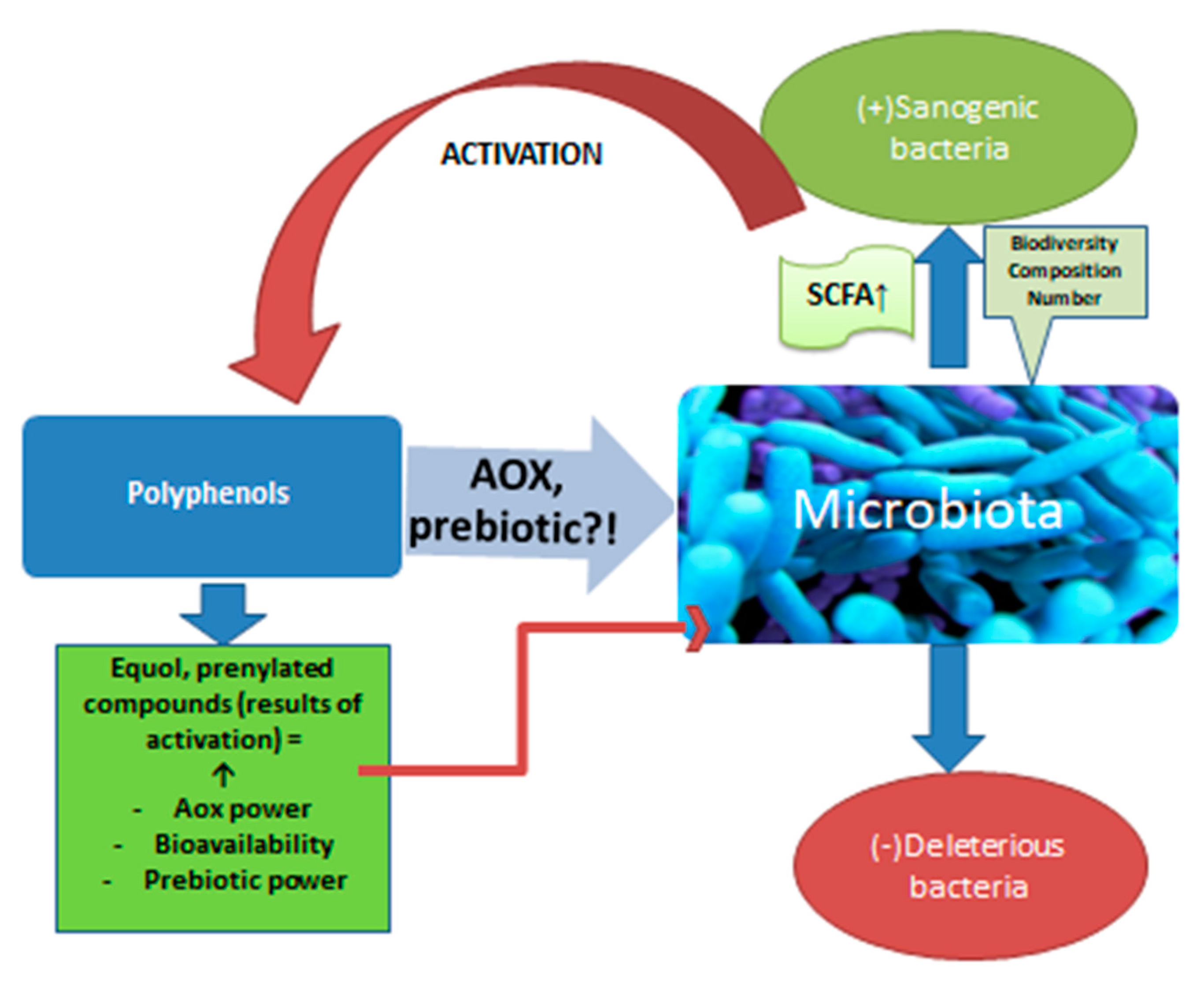
Figure 2. The action of polyphenols on the microbiota.
In a study conducted by Hernández-Quiroz et al. [45], after the administration of a dose of 355 mL of beer per day for 30 days in healthy subjects, divided into a group that received beer without alcohol (n = 35) and one that received beer with alcohol (n = 33), a clear influence on the intestinal microbiota, as well as on the functionality of pancreatic β cells and fasting blood glucose, could be observed. The action on the microbiota consisted of increasing the diversity of the microbiota, by favoring species of the Bacteroides type, at the expense of Firmicutes. The authors attribute this effect to the polyphenols in beer, and it was found in beer without alcohol. Beer with alcohol did not have positive effects of the same scope and negatively impacted blood sugar and β cell functionality.
Another observational study [46] found an increase in butyric acid, a byproduct of the intestinal microbiota, in beer consumers, but without quantifying the intake of polyphenols. Positive effects were found only with wine in a large study on twins in Great Britain, in which non-alcoholic beer was not an element of investigation [47]. A recent clinical study by Martínez-Montoro et al. [48] worked on adults aged 30–60, divided into two groups (with or without metabolic syndrome) who were administered beer with different concentrations of polyphenols, consumed successively, after respective washout periods. In the beginning there were no radical differences in microbiota characteristics between the two groups. During the study, the authors report significant changes in the microbiota, with substantial changes in the entire profile, all the more important as the polyphenol content of the beer was higher. The changes were also influenced by the metabolic status of the individuals, being significant in the group of subjects with metabolic syndrome, where the abundance of streptococcus was highest after consumption of dark beer. A previous study showed that certain species of streptococci interact with gallic acid and catechins, amplifying their antioxidant effects [43]. Moreover, some streptococci can transform beer melanoidins into an isoflavone with estrogenic and antioxidant action called equol [44]. The authors attribute the effects found in the group with metabolic syndrome to the correction of intestinal dysbiosis, which is usually present in individuals with the syndrome in question. Since the most important changes were found after the consumption of dark beer, very rich in antioxidant polyphenols, the authors explain the influence also through the respective antioxidant action on the microbiota, excluding the possible interference of alcohol, which was found in equal quantities in lager beer (with fewer polyphenols) and in the black one (with maximum level of polyphenols).
There are still open study perspectives in which to possibly follow the impact of some types of beer enriched in polyphenols, with or without alcohol, on the intestinal microbiota and from here, on the entire metabolism [4]. An extensive review of the effects of beer polyphenols on the microbiota was carried out by Quesada-Molina et al. [49]. The authors analyze the existing studies, noting that in principle a detailed analysis of the microbiota-polyphenols interaction is needed and that the existing results so far are based on deduction rather than on concrete quantification of the effects.
This entry is adapted from the peer-reviewed paper 10.3390/nu15040844
References
- Rosso, A.M. Beer and wine in antiquity: Beneficial remedy or punishment imposed by the Gods? Acta Med. Hist. Adriat. 2012, 10, 237–262.
- Bamforth, C.W. Nutritional aspects of beer—A review. Nutr. Res. 2022, 22, 227–237.
- Wang, J.; Jiang, L.; Sun, H. Early evidence for beer drinking in a 9000-year-old platform mound in southern China. PLoS ONE 2021, 16, e0255833.
- Ambra, R.; Pastor, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486.
- Zugravu, C.A.; Bohiltea, R.E.; Salmen, T.; Pogurschi, E.; Otelea, M.R. Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants 2022, 11, 241.
- Sohrabvandi, S.; Mortazavian, A.M.; Rezaei, K. Health-Related Aspects of Beer: A Review. Int. J. Food Prop. 2012, 15, 350–373.
- Díaz-Rubio, E.; Saura-Calixto, F. Dietary Fiber Complex in Beer. J. Am. Soc. Brew. Chem. 2009, 67, 38–43.
- Radu, M.C.; Boeru, C.; Marin, M.; Manolescu, L.S.C. SARS-CoV-2 Infection in Seven Childbearing Women at the Moment of Delivery, a Romanian Experience. Cureus 2021, 13, e12811.
- Cecarini, V.; Gogoi, O.; Bonfili, L.; Veneruso, I.; Pacinelli, G.; De Carlo, S.; Benvenuti, F.; D’Argenio, V.; Angeletti, M.; Cannella, N.; et al. Modulation of Gut Microbiota and Neuroprotective Effect of a Yeast-Enriched Beer. Nutrients 2022, 14, 2380.
- De Roos, J.; Van der Veken, D.; De Vuyst, L. The Interior Surfaces of Wooden Barrels Are an Additional Microbial Inoculation Source for Lambic Beer Production. Appl. Environ. Microbiol. 2018, 85, e02226-18.
- Marcos, A.; Serra-Majem, L.; Pérez-Jiménez, F.; Pascual, V.; Tinahones, F.J.; Estruch, R. Moderate Consumption of Beer and Its Effects on Cardiovascular and Metabolic Health: An Updated Review of Recent Scientific Evidence. Nutrients 2021, 13, 879.
- Tucker, K.L.; Jugdaohsing, R.; Powell, J.J.; Qiao, N.; Hannan, M.T.; Sripanyakorn, S.; Cupples, L.A.; Kie, D.P. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am. J. Clin. Nutr. 2009, 89, 1188–1196.
- Redond, N.; Nova, E.; Díaz-Prieto, L.E.; Marco, A. Effects of moderate beer consumption on health. Nutr. Hosp. 2018, 35, 41–44.
- Peng, M.Z.; Tabashsum, M.; Anderson, A.; Truong, A.K.; Houser, J.; Padilla, A.; Akmel, J.; Bhatti, S.O.; Rahaman, O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. 2020, 19, 1908–1933.
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270.
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R.A. Archaea Are Interactive Components of Complex Microbiomes. Trends Microbiol. 2018, 26, 70–85.
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019, 7, 14.
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589.
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Gordon, J.I. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227.
- Guigoz, Y.; Doré, J.; Schiffrin, E.J. The inflammatory status of old age can be nurtured from the intestinal environment. Curr. Opin. Clin. Nutr. Metab. Care. 2008, 11, 13–20.
- Dragomirescu, C.C.; Lixandru, B.E.; Coldea, I.L.; Palade, A.M.; Baltoiu, M.; Dinu, S.; Cristea, V.C.; Manolescu, L.; Popa, M.I. Comparative analysis of different phenotypic and molecular methods used for the taxonomic identification of Corynebacterium spp. isolated from clinical samples in Romania. Rom. Biotechnol. Lett. 2017, 22, 12926–12933.
- Das, S.; Deb, D.; Adak, A.; Khan, M.R. Exploring the microbiota and metabolites of traditional rice beer varieties of Assam and their functionalities. 3 Biotech 2019, 9, 174.
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236.
- Bongaerts, D.; De Roos, J.; De Vuyst, L. Technological and environmental features determine the uniqueness of the lambic beer microbiota and production process. Appl. Environ. Microbiol. 2021, 87, e00612-21.
- Ovalle-Marmolejo, X.Y.; Redondo-Solano, M.; Granados-Chinchilla, F.; Miranda-Castilleja, D.E.; Arvizu-Medrano, S.M. Effect of stress factors on the production of biogenic amines by lactic acid bacteria isolated from fermented Mexican foods (cheese and beer). Food Control 2023, 146, 109553.
- Rodríguez-Saavedra, M.; de Llano, D.G.; Moreno-Arribas, M.V. Beer spoilage lactic acid bacteria from craft brewery microbiota: Microbiological quality and food safety. Food Res. Int. 2020, 138 Pt A, 109762.
- Fossi, B.T.; Ekabe, D.E.; Toukam, L.L.; Tatsilong Pambou, H.O.; Gagneux-Brunon, A.; Nguefeu, C.N.; Bongue, B. Probiotic lactic acid bacteria isolated from traditional cameroonian palm wine and corn beer exhibiting cholesterol lowering activity. Heliyon 2022, 8, e11708.
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.I.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102.
- Kordialik-Bogacka, E. Biopreservation of beer: Potential and constraints. Biotechnol. Adv. 2022, 58, 107910.
- Bossaert, S.; Winne, V.; Van Opstaele, F.; Buyse, J.; Verreth, C.; Herrera-Malaver, B.; Van Geel, M.; Verstrepen, K.J.; Crauwels, S.; De Rouck, G.; et al. Description of the temporal dynamics in microbial community composition and beer chemistry in sour beer production via barrel ageing of finished beers. Int. J. Food Microbiol. 2021, 339, 109030.
- Miguel, G.A.; Carlsen, S.; Arneborg, N.; Saerens, S.M.C.; Laulund, S.; Knudsen, G.M. Non-Saccharomyces yeasts for beer production: Insights into safety aspects and considerations. Int. J. Food Microbiol. 2022, 383, 109951.
- Takagak, A.; Nanjo, F. Bioconversion of (−)-Epicatechin, (+)-Epicatechin, (−)-Catechin, and (+)-Catechin by (−)-Epigallocatechin-Metabolizing Bacteria. Biol. Pharm. Bull. 2015, 38, 789–794.
- Ou, J.; Huang, J.; Song, Y.; Yao, S.; Pen, X.; Wang, M.; Ou, S. Feruloylated Oligosaccharides from Maize Bran Modulated the Gut Microbiota in Rats. Plant Foods Hum. Nutr. 2016, 71, 123–128.
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422.
- Queipo-Ortuñ, M.I.; Boto-Ordóñez, M.; Murri, M.; Gómez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334.
- Ghiselli, A.; Natella, F.; Guidi, A.; Montanar, L.; Fantozzi, P.; Scaccini, C. Beer increases plasma antioxidant capacity in humans. J. Nutr. Biochem. 2000, 11, 76–80.
- Possemiers, S.; Heyerick, A.; Robbens, V.; De Keukeleire, D.; Verstraete, W. Activation of Proestrogens from Hops (Humulus lupulus L.) by Intestinal Microbiota; Conversion of Isoxanthohumol into 8-Prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6628.
- Moreno Indias, I. Beneficios de los polifenoles contenidos en la cerveza sobre la microbiota intestinal . Nutr. Hosp. 2017, 34, 41–44.
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 15, 905215.
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly Cancer Selective Antiproliferative Activity of Natural Prenylated Flavonoids. Molecules 2018, 23, 2922.
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 2, 78.
- Khalil, R.K.S. Influence of gallic acid and catechin polyphenols on probiotic properties of Streptococcus thermophilus CHCC 3534 strain. World J. Microbiol. Biotechnol. 2010, 26, 2069–2079.
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Ángel Rufián-Henares, J. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020, 316, 126309.
- Hernández-Quiroz, F.; Nirmalkar, K.; Villalobos-Flores, L.E.; Murugesan, S.; Cruz-Narváez, Y.; Rico-Arzate, E.; Hoyo-Vadillo, C.; Chavez-Carbajal, A.; Pizano-Zárate, M.L.; García-Mena, J. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and β-cell function. Alcohol 2020, 85, 77–94.
- González-Zancada, N.; Redondo-Useros, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A.; Nova, E. Association of Moderate Beer Consumption with the Gut Microbiota and SCFA of Healthy Adults. Molecules 2020, 25, 4772.
- Le Roy, C.I.; Wells, P.M.; Si, J.; Raes, J.; Bell, J.T.; Spector, T.D. Red Wine Consumption Associated with Increased Gut Microbiota α-Diversity in 3 Independent Cohorts. Gastroenterology 2020, 158, 270–272.
- Martínez-Montoro, J.I.; Quesada-Molina, M.; Gutiérrez-Repiso, C.; Ruiz-Limón, P.; Subiri-Verdugo, A.; Tinahones, F.J.; Moreno-Indias, I. Effect of Moderate Consumption of Different Phenolic-Content Beers on the Human Gut Microbiota Composition: A Randomized Crossover Trial. Antioxidants 2022, 11, 696.
- Quesada-Molina, M.; Muñoz-Garach, A.; Tinahones, F.J.; Moreno-Indias, I. A New Perspective on the Health Benefits of Moderate Beer Consumption: Involvement of the Gut Microbiota. Metabolites 2019, 9, 272.
This entry is offline, you can click here to edit this entry!
