Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Withaferin A, a natural bioactive molecule isolated from the Indian medicinal plant Withania somnifera (L.) Dunal, has been reported to impart anticancer activities against various cancer cell lines and preclinical cancer models by modulating the expression and activity of different oncogenic proteins.
- withaferin A
- cancer
- apoptosis
- angiogenesis
- chemoresistance
1. Structure of Withaferin A
The bioactive compound withaferin A is a steroidal lactone primarily extracted from W. somnifera, first isolated by Israeli chemists, Asher Lavie and David Yarden, in 1962 from the leaves of the plant. The inhibitory properties of the withaferin A molecule are due to the occurrence of an unsaturated lactone in a side chain; allylic 1° alcohol is attached at position 25 and the highly oxygenated rings A and B are attached to the other side of the compound [32,33,34,35,36]. Cytotoxicity is achieved by the presence of α,β-unsaturated carbonyl moiety of the withaferin A structure of thiol adducts [37]. Withaferin A structural studies revealed that the three positions susceptible to nucleophilic attack are the unsaturated A-ring at C3, the epoxide at position five, and C24 in the E ring (Figure 1). All these sites of withaferin A are covalently attached to the cysteine residues of protein by the Michael addition alkylation reaction, resulting in a loss of the activity of the target protein [38]. However, the C27 hydroxy group is not biologically important for withaferin A activity and can be conjugated with biotin so that it may recognize various target proteins [39,40]. Withaferin A was first isolated from the ether extract of Withania plant leaves and analyzed by thin-layer chromatography (TLC) and column chromatography. The initial extraction of withaferin A was performed by repeated column chromatography using the chloroform–methanol fraction of the W. somnifera extract and partial purification of the compound using TLC and high-performance liquid chromatography. In the end, nuclear magnetic resonance and Fourier transform infrared were used to characterize the product [41,42].
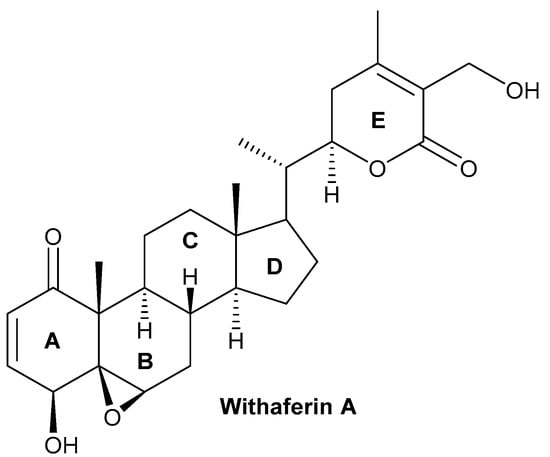
Figure 1. Chemical structure of withaferin A.
Moreover, withaferin A may also be extracted from the root of W. frutescens, a plant native to Europe with a high content of withaferin A in its leaves, which has shown a similar phytochemical profile to W. somnifera [43]. In another study, in vitro and in situ grown W. somnifera plants underwent morphological and phytochemical screening for the optimization of withaferin A extraction. The highest amount of withaferin A obtained under in vitro conditions was a concentration of 0.27 to 7.64 mg/g dry weight, while under in situ conditions the concentration was 8.06 to 36.31 mg/g dry weight [44]. The evaluation of structure–activity relationships in 56 withanolides using 2D and 3D coculture models revealed withanolide D (2) as a compound that has high antiproliferative activity against multiple myeloma [45].
2. Biosynthesis of Withanolides
Withaferin A contains five important chemical groups: an epoxide ring between carbons 5 and carbon 6, a hydroxyl group at carbon 27, a 6-membered lactone ring (E) with an α, a β-unsaturated carbonyl group, an α,β-unsaturated ketone group in ring A, and a secondary hydroxyl group at carbon 4. Several studies have shown that changing a few of the aforementioned chemical groups aids in the synthesis of bioactive semisynthetic analogs of withaferin A [46,47,48,49].
Various types of withanolides are synthesized through mevalonate and non-mevalonate routes often known as the isoprenoid pathway and a range of enzymes, such as cycloartenol synthase, (S)-2, 3-epoxysqualene mutase belongs to the 2–3 oxidosqualene cyclases (OCSs) gene family along with α-amyrin synthase (AAS), β-amyrin synthase (BAS), lupeol synthase (LS), and lanosterol synthase (LAS) [50,51]. Withanolides are formed biosynthetically by cycloartenol synthase (CAS). The cyclization of 2,3-oxidosqualene into cycloartenol by the enzyme CAS is a crucial step in the formation of all withanolides in W. somnifera plants. A step-by-step process that includes desaturation, hydroxylation, epoxidations, cyclization, chain elongation, and glycosylation, is preceded by cycloartenol molecules that serve as a precursor for the synthesis of withanolides. The biosynthesis of withanolides began with the cyclization of epoxysqualene to produce the triterpenoid compound C30H50O.
Plants contain various types of oxidosqualene cyclases that are essential for the synthesis of sterols and many types of triterpenoids from 2,3-oxidosqualene. In the first step of mevalonate pathways, the enzyme acetoacetyl (AcAc)-CoA thiolase is involved in the condensation of two acetyl CoA molecules into AcAc-CoA to form 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) [52]. In the second step, AcAc-CoA is condensed with one molecule of acetyl-CoA to form HMG-CoA with the help of an enzyme HMG-CoA synthase [53]. In the third step, a nicotinamide adenine dinucleotide (phosphate)-dependent (NAD(P)H) enzyme and HMG-CoA reductase biosynthesized mevalonate (MVA) from HMG-CoA (Benveniste, 2002). MVA is converted further into isopentyl pyrophosphate (IPP) via phosphorylation and decarboxylation, which are carried out by a series of enzymes, including MVA kinase, phosphomevalonate kinase, and MVA diphosphate decarboxylase. The IPP resulting from the cytosolic MVA pathway changes into dimethylallyl diphosphate (DMAPP) (Hunter, 2007). The IPP and DMAPP are further involved in the formation of farnesyl pyrophosphate (FPP), which is the main precursor for the synthesis of triterpenoids [54,55].
When withanolides are produced by the methylerythritol 4-phosphate (MEP) route, an enzyme 1-deoxy-D-xylulose 5-phosphate synthase converts the major substrates, pyruvate, and glyceraldehydes 3-phosphate, into 1-deoxy-D-xylulose 5-phosphate (DXP) [56,57]. In the next step, the DXP changes into the MEP and it further changes into the 1-hydroxy-2-methyl-2-€- butenyl 4-diphosphate, with the help of enzymes DXP reductoisomerase and successive action of other enzymes, such as 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase, 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase, 2-C- methyl-D-erythritol 2,4-cyclodiphosphate synthase, and (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (HMBPP). The last step of this pathway involved the branching of HMBPP to IPP and DMAPP catalyzed by an enzyme, (E)-4-hydroxy-3-methyl but-2-enyl diphosphate reductase. The IPP further leads the development of the main precursor of FPP for triterpenoids biosynthesis [54,55]. The FPP reaction is promoted into the condensation of IPP with DMAPP to form 10-C intermediate geranyl diphosphate (GPP), and the condensation of GPP with IPP results in 15-C FPP [58]. Squalene, which is produced by the action of the enzyme squalene synthase, which catalyzed the condensation of two molecules of FPP in NADPH, is one of the most important molecules for the biosynthesis of triterpenoids. Now that the squalene has undergone epoxidation to create squalene 2,3,-epoxide, cycloartenol has been biosynthesized, and it has subsequently evolved into a range of different kinds of steroidal triterpenoidal compounds [58,59,60].
3. Anti-Inflammatory and Antioxidant Activities of Withaferin A
The uncontrolled regulation of various inflammatory markers, such as chemokines and cytokines, plays a key role in the inflammatory process [161,162,163,164,165,166]. Inflammation results in the release of many free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), which cause oxidative stress and contribute to the development of various pathological conditions, such as atherosclerosis, cardiovascular disease, and cancer [167,168,169,170,171,172,173]. Cancer growth, progression, and chemotherapy treatment can all induce inflammation in cancer patients [174,175,176,177,178]. Therefore, as compared to synthetic drugs and formulations, natural bioactive metabolites that target different kinds of cancer may substantially alleviate side effects and offer new alternatives to the standard of care for cancer patients [179,180,181,182]. Currently, only a few bioactive compounds isolated from various natural sources have been tested clinically for cancer treatment, and one of these bioactive compounds, withaferin A, isolated from W. somnifera, has potential anticancer, anti-inflammatory, and antioxidant properties [183,184,185].
Withaferin A has shown cytotoxic properties against various cancers, such as leukemia, liver, oral, colon, pancreas, prostate, breast, ovarian, and bladder cancer [186,187]. The anti-inflammatory qualities of withaferin A are specifically attributed to its inhibition of pro-inflammatory molecules, α-2 macroglobulin, NF-κB, activator protein 1 (AP-1), and cyclooxygenase-2 (COX-2) inhibition, observed in various in vitro models [188]. Human and mouse islets treated with withaferin A demonstrated the inhibition of NF-ĸB signaling, preventing cytokine-induced death by reducing the secretion of cytokines, thereby effectively protecting the islet [189] (Figure 3).
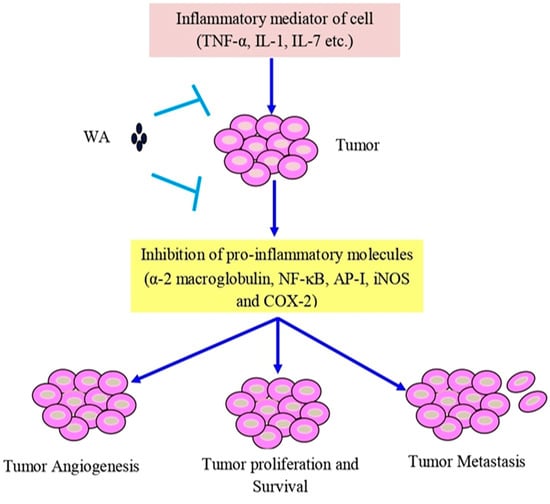
Figure 3. Withaferin A intercedes in anti-inflammatory function in tumor cells via TNF-α, IL-1, and IL-7. Abbreviations: IL-1, interleukin-1; IL-7, interleukin-7; and TNF-α, tumor necrosis factor-α.
In another study, it was observed that the activation of the Toll-like receptor 4 (TLR4) in spinal cord astrocytes triggers a signal flow that leads to the commencement of NF-ĸB, which further initiates the expression of TNF-α, COX-2, and inducible nitric oxide synthase (iNOS), and pro-inflammatory and stress-response moderators that may cause CNS disorders, such as neural cell death. However, withaferin A is highly effective in inhibiting the transcriptional activity of NF-ĸB and pro-inflammatory and stress-response mediators in astrocytes, exhibiting the potential for withaferin A to combat neurodegenerative disorders [190,191,192,193,194]. Other studies also revealed that withaferin A reduced NADPH oxidase as well as superoxide levels, which prevented the aging-induced neurodegeneration of the dopaminergic neurons in the brain of a rat model [195,196].
Withaferin A also acts as a key mediator in the prevention of inflammation during chronic kidney disease (CKD), seen in the unilateral urethral obstruction (UUO) renal injury animal model (unilateral obstruction). The levels of TGF-β and downstream signaling molecules p-Smad2, p-Smad3, total Smad4, p-Akt, and p-ERK were attenuated by withaferin A, showing strong evidence of the renoprotective potential of withaferin A due to its anti-inflammatory activity [197]. Withaferin A can also display antioxidant effects in liver fibrosis, by attenuating the BB-(PDGF-BB) platelet growth factor and promoting PDGF-BB-induced SIRT3 expression and action in the case of JS1 cells. It also prevented carbon tetrachloride (CCl4)-induced liver damage, fibrosis, and collagen deposition by increasing the sirtuin3 (SIRT3) expression and suppressing CCl4-induced oxidative stress in the fibrotic liver of C57/BL6 mice [198]. Studies have shown that withaferin A is capable of restoring the structure of the liver by increasing antioxidant action in hepatocarcinogenic rats by lowering the level of liver marker enzymes and reducing the oxidative stress of various oxidants [199]. Another study observed that withaferin-A activated LXR-α, which inhibits NF-κB transcriptional activity and suppresses the proliferation, invasion, migration, and anchorage-independent growth of hepatocellular carcinoma (HCC) cells, confirming withaferin A to be a potent anticancer compound that suppresses various angiogenesis and inflammatory markers linked to the development and progression of HCC.
Interestingly, low concentrations of withaferin A treatment for 24 h did not show cytotoxicity against Ca9–22 oral cancer cells, but did cause the release of ROS, wound healing, and the migration of cells. At the molecular level, withaferin A inhibits matrix metalloproteinase-2 (MMP-2) and MMP-9, but also provokes mRNA stimulation for a set of antioxidant genes, such as NADPH quinone dehydrogenase 1 (NQO1), glutathione-disulfide reductase (GSR), Nrf2, heme oxygenase 1 (HMOX1), and induced mild phosphorylation in the MAPK family, including extracellular signal-regulated kinases 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 expression in Ca9–22 cells. All these alterations were suppressed by the presence of the ROS scavenger N-acetylcysteine (NAC), suggesting that low concentrations of withaferin A can maintain potent ROS-mediated antimigration and invasion capabilities of oropharyngeal squamous cancer cells [200]. Withaferin A improved the ability of H9c2 cells to survive against simulated ischemia/reperfusion (SI/R) or hydrogen peroxide (H2O2)-induced cell death in a cardiac ischemia-reperfusion injury model. Withaferin A triggered the upregulation of superoxide dismutase SOD2, SOD3, and peroxiredoxin 1(Prdx-1). Additionally, withaferin A inhibited the H2O2-induced upregulation of SOD2, SOD3, and Prdx-1, and ameliorated cardiomyocyte caspase-3 activity via an Akt-dependent pathway [201].
Chaudhary et al. [202] reported that an overproduction of ROS accelerated by withaferin A was responsible for the inhibition of the cell cycle in CRC cells and that it decreased the potential of the mitochondrial membrane, causing mitochondrial dysfunction. Withaferin A promoted radiation-induced apoptosis in human kidney carcinoma (Caki) cells by producing reactive oxygen species and inhibiting Bcl-2 and Akt dephosphorylation [16]. Withaferin A, in combination with doxorubicin (DOX), is also responsible for the excessive generation of ROS that can cause concentration-dependent DNA damage and stimulate autophagy in an ovarian cancer cell line (Figure 4). The histochemical observation of tumor tissues treated with withaferin A and DOX showed a reduction in the cell proliferation and micro-vessel development, an increase in light chain 3β (LC3B) levels, DNA destruction, and the cleavage of caspase-3 [203,204,205].
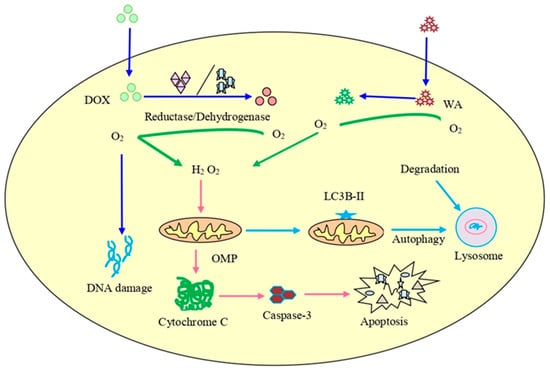
Figure 4. The combined treatment of doxorubicin (DOX) and withaferin A on cancer cells. It can lead to enhanced ROS production, destruction of DNA, initiation of autophagy, and increased expression of LC3B autophagy marker as well as cleavage of caspase-3.
4. Safety and Toxicity of Withaferin A
During the review of the bioactive compound withaferin A, it was recognized that one of its most important attributes is its safety for normal cells and tissues, which allows for the continuation of this drug in clinical trials. In an in vivo study, W. somnifera extract containing withaferin A was orally administered to Wistar rats at a dose of 2000 mg/kg/day and had no adverse effects on the animals [255]. Moreover, withaferin A is also responsible for the reduction of acetaminophen-induced liver toxicity in mice through the stress-responsive transcription factor Nrf2. Similarly, another study found that withaferin A increased the expression of SIRT3 and suppressed CCl4-induced oxidative stress in the fibrotic liver of a C57/BL6 mice model, assisting in reduced liver injury, collagen deposition, and fibrosis caused by CCl4 [198]. Withaferin A was also shown to decrease severe cerulein-induced pancreatitis caused by inflammation and oxidative stress. The treatment of scleroderma with withaferin A initiated antifibrotic activity, which was repressed by the proinflammatory fibrosis related to TGF-β/Smad signaling and the FOXO3a-Akt-dependent NF-κβ/IKK-mediated inflammatory mechanism [256,257,258,259,260]. In addition, withaferin A has also exerted potential cytotoxicity effects at a low range (up to 5 μM) in melanoma cells, as compared to non-malignant cells [155]. Withaferin A significantly inhibited the burden of breast cancer in two different subtypes, HER2-driven breast cancer, and luminal-type breast cancer-bearing rodent models, when administered through the intraperitoneal route [261]. Most of the findings related to the general safety of withaferin A support the pharmacological and pharmaceutical studies of this natural bioactive compound. However, more research and pharmacokinetic studies of withaferin A and its derivatives are required for the development of chemotherapeutic drugs in the future.
5. Clinical Studies of Withaferin A
Based on impressive preclinical results, the potential of withaferin to treat a wide range of disorders is significant. However, presently there is only one ongoing clinical trial to examine the efficacy of withaferin A in cancer patients. A phase-I/II clinical trial is initiated in which the combination of DOXIL and withaferin A is examined in patients with recurrent ovarian cancer. This study aims to determine the feasibility and maximum tolerance dose of withaferin A with DOXIL and to examine the complete response, partial response, and stable disease (NCT05610735). Although there is a huge amount of preclinical data available on the anticancer potential of withaferin A, these studies are yet to substantiate its antitumor efficacy in the clinical setting. Therefore, withaferin A needs to be investigated rigorously in randomized controlled trials to understand its therapeutic efficacy against human malignancies.
This entry is adapted from the peer-reviewed paper 10.3390/ph16020160
This entry is offline, you can click here to edit this entry!
