Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Tuberculosis (TB) prevalence is increasing in developed nations and continuing to cause significant mortality in low- and middle-income countries. As a result of the uptick in cases, there also exists an increased prevalence of extrapulmonary TB. TB is caused by Mycobacterium tuberculosis (M. tb). When M. tb disseminates to the vertebral column, it is called Pott’s disease or spinal TB. The frequency, symptoms, and severity of the disease range by the location of the spine and the region of the affected vertebrae.
- M. tb
- Pott’s disease
- spinal TB
- extrapulmonary TB
- pathogenesis
1. Introduction
Mycobacterium tuberculosis (M. tb) is the second leading cause of death from an infectious agent worldwide [1]. In 2018, approximately 10 million people became infected with TB, and 1.5 million died due to TB [2]. Primary infection by M. tb often seeds itself in the lungs of its host, causing Tuberculosis (TB), though in rare instances, the infection may spread to the bone and joints. Skeletal TB comprises 10% of extrapulmonary TB cases, of which 50% involve the spinal column [3][4]. Spinal TB is otherwise known as Pott’s disease, named after Pervical Pott, the first patient to present with classic spinal TB in 1779 [3][5].
The variety of the clinical manifestations of Pott’s disease proves to be a significant challenge in providing a timely and correct diagnosis in both developed and developing countries. Despite advances in imaging modalities, the time between the initial onset of symptoms to the time of diagnosis ranges between four and six months [6][7]. A delay in diagnosis is the factor that most contributes to a worse prognosis with an increased likelihood of surgical treatment and neurological deficits [6][7][8][9][10]. Magnetic resonance imaging (MRI) is capable of identifying the soft tissue changes in spinal TB but is often employed at the time of neurological symptom presentation and is mistaken for nontuberculous spinal infection [3][11]. Once symptoms progress to neurological deficits, a significant number of patients may never recover their neurological function. Kumar et al. revealed that their surgical center noted that 28% of total pediatric cases remained the same or worse after surgery, with the majority of patients presenting with neurological symptoms [12]. Treatment at the early stages of Pott’s disease with the standard treatment regimen leads to healing in approximately 95% of patients [13]. Therefore, early diagnosis is crucial to prevent the development of sequelae. However, physicians often exhibit a low index of suspicion of Pott’s disease, especially those from low-incidence countries [6]. Patients may present with non-specific symptoms and misleading data, such as a negative purified protein derivative (PPD) skin test despite active infection [3][14]. To avoid delays in diagnosis, there must be increased awareness of the diverse clinical presentations as well as improvements in the diagnostic criteria of Pott’s disease.
In addition to identifying key diagnostic modalities for the detection of Pott’s disease, it is crucial to understand the risk factors in patient populations to identify at-risk individuals and to expand upon the prophylactic measures to reduce the incidence of the disease. Immunodeficiency, most notably HIV, as well as vitamin D deficiency, are strongly implicated as risk factors associated with Pott’s disease due to their profound influence on the body’s ability to handle the infective processes of M. tb [15][16].
Pott’s disease has surged in developed nations, especially targeting immunocompromised individuals, due to global migration [17].
2. Pathogenesis and Clinical Presentation of Pott’s Disease
With the recent uptick in the incidence of TB in both developed and developing countries, having an understanding of the rare presentations of TB is increasing in importance [3]. M. tb spreads through aerosol droplets to cause a primary infection in the lungs of the host due to its dependence on oxygen [18]. As seen in Figure 1, M. tb infection has multiple avenues in which it spreads throughout the host [19]. Without proper treatment or detection, the infection may become latent in the lungs or spread via hematogenous dissemination to the extrapulmonary sites of the body [18][20].
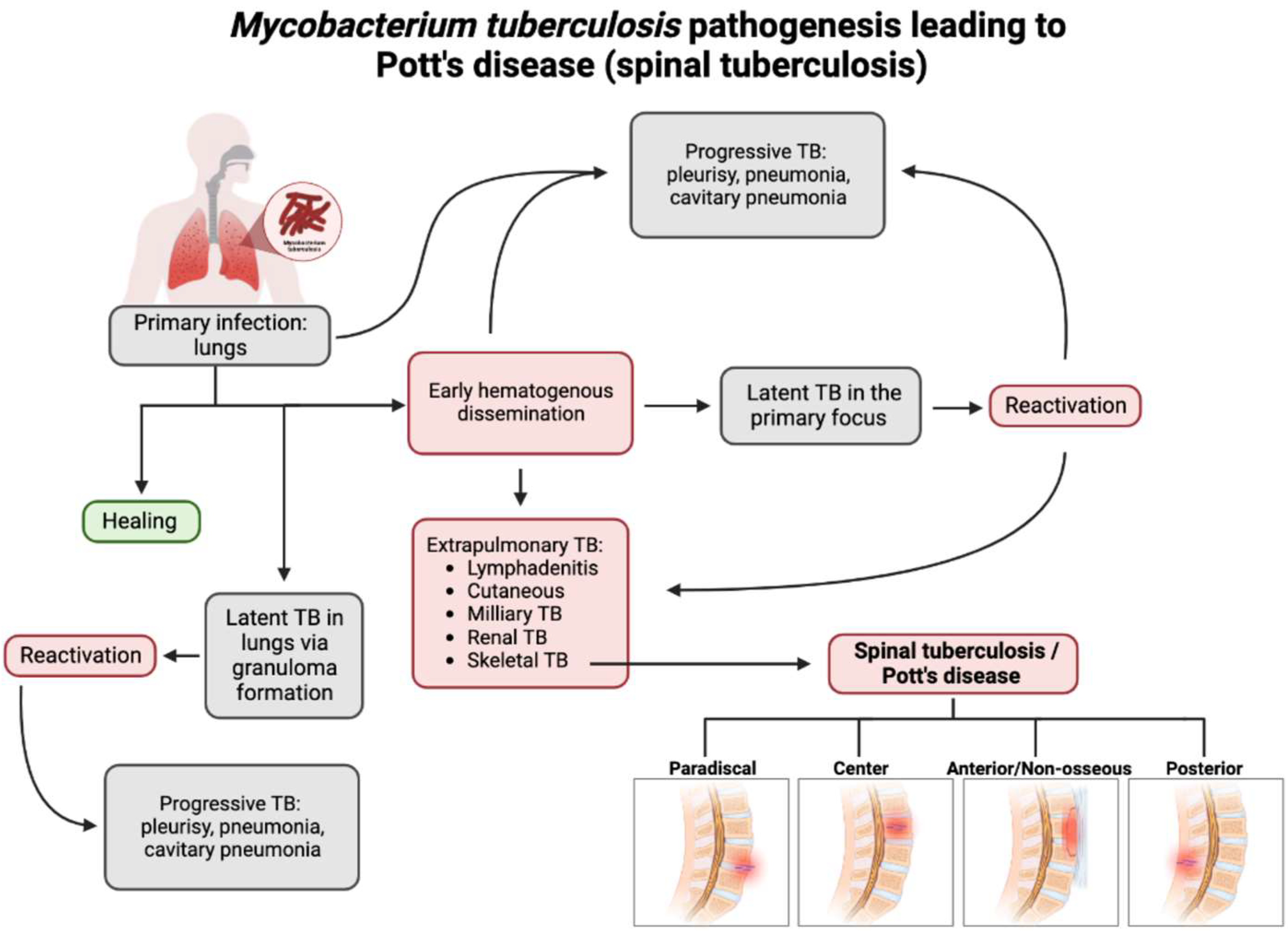
Figure 1. Flow chart depicting the pathogenesis of Pott’s disease. From primary infection, spinal tuberculosis may develop from hematogenous dissemination from the primary infection site and spread to an extrapulmonary site, one of which is the involvement of the skeletal system and, thus, the spine. Following this infection of the spine, spinal TB could present in four major presentations: paradiscal (involves the subchondral space and disc degeneration), central (stems from the center of the vertebral body), anterior/non-osseous (abscess formation between the spinal column and the anterior longitudinal ligament, often spanning multiple segments, sparing bone/disc involvement), and posterior (the infection seeds in the neural arch and posterior aspects of the vertebra). This leads to a compromise and the destruction of the spinal column and causes the classic and atypical presentations of spinal TB/Pott’s disease [3][18][19][20].
Spinal TB encompasses 2% of all TB cases, 15% of extrapulmonary cases, and 50% of skeletal TB cases [5]. Hematogenous dissemination is the primary mechanism by which spinal TB arises [20]. Spinal TB arises from a primary focus from the classic pulmonary focus or another extraosseous focus, such as the lymph nodes, GI tract, or another visceral location in which mycobacteria have infiltrated [5][18][20]. Approximately 67% of spinal TB cases are linked to primary pulmonary TB [4]. These foci could have active, subclinical, or quiescent disease [5][21]. For example, Jung et al. demonstrated a case of spinal TB originating from latent TB infection in a pediatric patient being treated for Crohn’s disease with infliximab. The patient developed miliary TB one month after infliximab initiation and was subsequently cured of miliary TB; however, the patient developed spinal TB three months later [22].
After dissemination, the bacteria travel through the vascular system, using the anterior and posterior spinal arteries of each vertebra, to ultimately reach the cancellous bone region of the vertebra, with the most common site being the thoracolumbar junction [4][23]. Using the valveless Batson’s paravertebral venous plexus, intrabdominal and intrathoracic pressures spread the infection to the inferior anterior portion of a vertebral body [5][18]. From here, the infection may utilize the anterior longitudinal ligament to infect adjacent vertebrae, as seen in Figure 2 [21]. Paradiscal and central are among the more common presentations of spinal TB, along with anterior/non-osseous and posterior lesions [4][5][20].
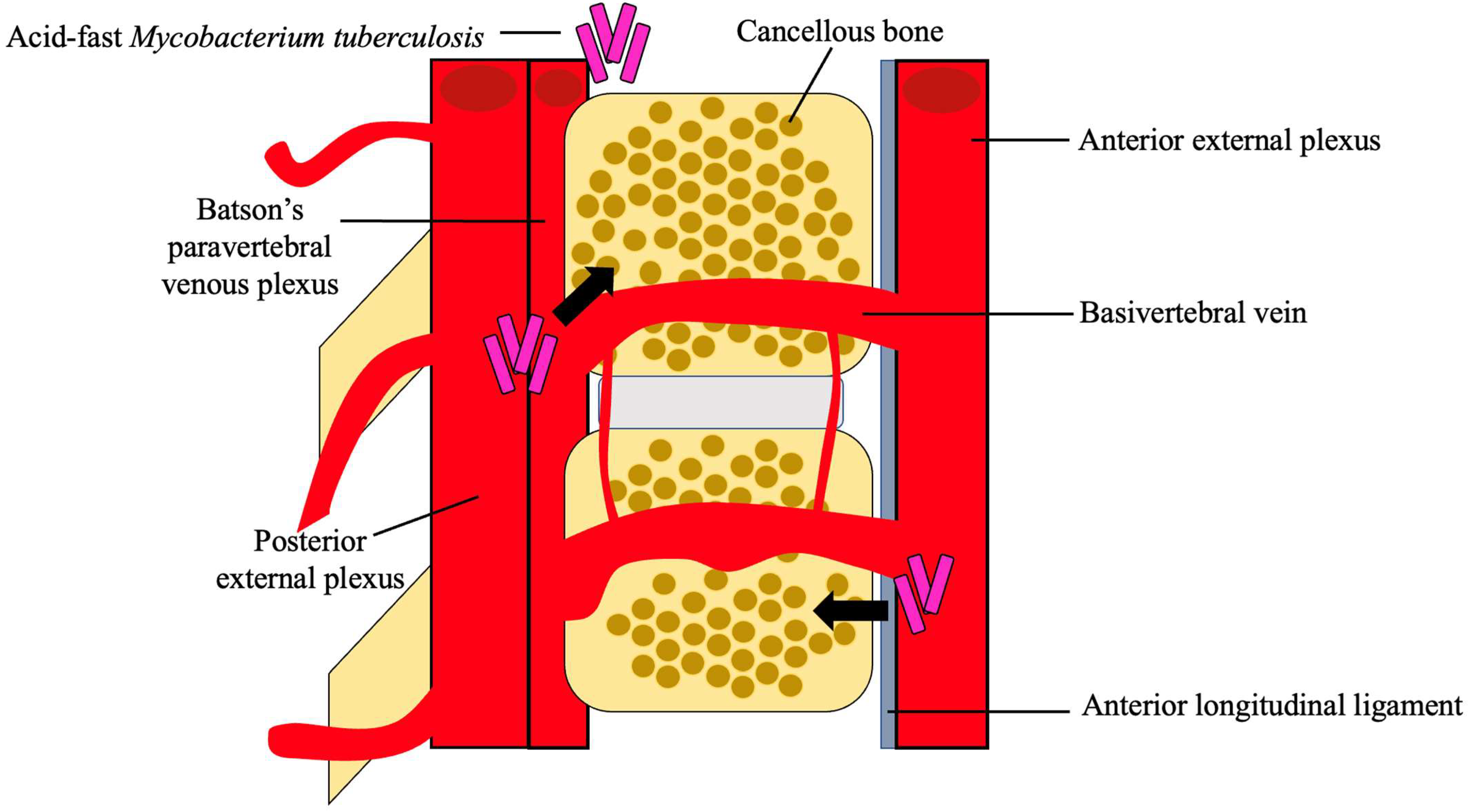
Figure 2. Hematogenous spread of M. tb. M. tb reaches the cancellous bone through the anterior and posterior external venous plexuses. A combination of the valveless Batson’s paravertebral venous plexus and cavity pressures spreads M. tb to the inferior anterior portion of the vertebra and ultimately to the anterior longitudinal ligament where it may disseminate to adjacent vertebra [4][5][18][21].
For paradiscal involvement, the most common type of lesion, M. tb lodges into the subchondral marrow of the vertebra leading to disc destruction [5][18][20]. This disc destruction leads to an anterior wedge of the involved vertebrae creating the characteristic kyphosis of Pott’s disease. This type of spinal TB also often presents with intraosseous and extraosseous abscess formation, which places the patient at an increased risk for spinal cord damage and possible paraplegia [5]. These lesions are more likely to occur in younger patients due to the increased vascularization in pediatric paravertebral discs compared to adults [4].
For central involvement, M. tb infects the center of the vertebral body, leading to the destruction of the vertebral body itself, often sparing the disc [5][20]. This type of lesion leads to the complete compression of the vertebral body, known as vertebra plana [4]. Garg et al. demonstrated in a 5-year retrospective study of 1,652 patients in India that 82% of spinal TB patients had the paradiscal type of lesions, and 15.2% had the central type of lesions [24].
Anterior or non-osseous involvement initially spares the bone and disc of the spinal column and creates an abscess using the anterior longitudinal ligament to spread over multiple contiguous vertebrae [5][18][20]. These abscesses are granulomatous in nature, and as they grow, the periosteum lifts, leading to bone devascularization and, eventually, necrosis and deformity [18][20]. Posterior involvement follows similar pathogenesis to anterior involvement yet utilizes the posterior longitudinal ligament and often involves the neural arch [18][20]. Paradiscal, central, and non-osseous-type lesions represent approximately 98% of all spinal TB cases, showing that posterior-type lesions are much less common [25].
Should the infection lead to bone collapse or spinal canal compromise, the infection would then be classified as TB spondylitis [26]. At this stage, the clinical findings may be hard to differentiate from a pyogenic or fungal osteomyelitis of the spine or metastatic bone tumors [26]. Given the potential level of destruction that spinal TB could lead to, it is important to understand the classical clinical presentation of the infection.
Spinal TB more frequently presents in children and young adults due to the increased vascularization of their spine, often with paradiscal lesions and in immunosuppressed patients [3][4]. Classically, spinal TB presents with pain and tenderness over the spine, neurological deficits, cold abscesses, fevers, and, if found in a later stage, a kyphotic spinal deformity and instability, as depicted in Figure 3 [3][5][20]. This presentation, however, depends on the duration of the disease, the severity of spinal destruction, and the site of the infection [3]. Spinal TB more frequently infects the lower thoracic and lumbar region but could present in the cervical spine as well [4][20]. Additionally, it typically presents without the symptoms of pulmonary TB, although both could be simultaneously present [20].
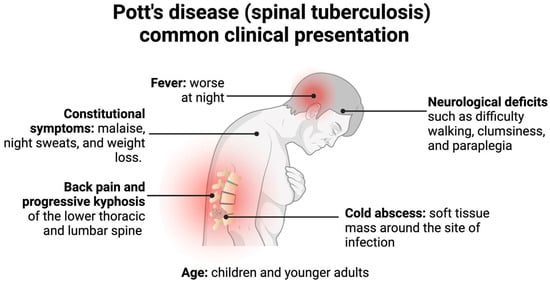
Figure 3. Pott’s disease commonly presents in children or younger adults with back pain, neurological deficits, cold abscesses, and possible kyphotic deformities. Less commonly, patients present with constitutional symptoms and fevers, which generally precede the former symptoms [3][4][5][19][20][25][26].
The earliest and most common of these symptoms is back pain, which is associated with localized swelling and tenderness with progressive paraspinal muscle spasms, leading to restriction and pain in all planes of spinal motion [5]. In the later stages of the infection, back pain presents with an associated kyphosis of the affected spinal area [4]. This associated kyphosis could continue despite the resolution of the infection. In adults, kyphosis is limited to active disease, but in children, kyphosis could increase or worsen in periods of growth, further perpetuating other associated symptoms of Pott’s disease, such as neurological deficits and possible paraplegia [3][20].
The second most common associated clinical symptom is neurological deficits, determined by the level at which the spine is affected [4]. Thus, an infection site in the cervical region would present with neurological deficits indicative of both upper extremity and lower extremity dysfunction, whereas a lumbar site of infection would only present with lower extremity and sacral deficits [4]. These deficits are a consequence of direct neural compression, the invasion of the neural parenchyma, tuberculous meningitis, pathological dislocation and the subluxation of the vertebrae, or vascular compromise to the spinal cord segment [18]. Since the infection starts at the anterior portion of the vertebrae, neurological deficits would work their way from anterior to posterior, starting with involvement of the anterior spinal tract, leading to exaggerated tendon reflexes and upper motor neuron-type deficits [3]. This could progress to a weakness of the limbs and difficulty with ambulation due to muscle spasms [25]. Left untreated, this further progresses to bladder and bowel dysfunction, sensory loss, and possible paraplegia [3].
Cold abscesses are also commonly associated with spinal TB and are typically located near the initial lesion [4]. A cold abscess denotes an abscess in which there are no inflammatory indications, such as warmth or erythema [3]. These abscesses may grow to be very large and add to the symptoms the patient presents with. For example, if the initial lesion is in the cervical region, the cold abscess could be formed in the retropharyngeal pouch and produce pressure effects that cause dysphagia, respiratory distress, or hoarseness of the voice [4].
This entry is adapted from the peer-reviewed paper 10.3390/clinpract13010014
References
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- MacNeil, A.; Glaziou, P.; Sismanidis, C.; Date, A.; Maloney, S.; Floyd, K. Global Epidemiology of Tuberculosis and Progress Toward Meeting Global Targets—Worldwide, 2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 281–285.
- Viswanathan, V.K.; Subramanian, S. Pott Disease. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022.
- Garg, R.K.; Somvanshi, D.S. Spinal tuberculosis: A review. J. Spinal Cord Med. 2011, 34, 440–454.
- Ansari, S.; Amanullah, M.F.; Ahmad, K.; Rauniyar, R.K. Pott’s Spine: Diagnostic Imaging Modalities and Technology Advancements. N. Am. J. Med. Sci. 2013, 5, 404–411.
- Colmenero, J.D.; Ruiz-Mesa, J.D.; Sanjuan-Jimenez, R.; Sobrino, B.; Morata, P. Establishing the diagnosis of tuberculous vertebral osteomyelitis. Eur. Spine J. 2013, 22 (Suppl. 4), 579–586.
- Kamara, E.; Mehta, S.; Brust, J.C.M.; Jain, A.K. Effect of delayed diagnosis on severity of Pott’s disease. Int. Orthop. 2012, 36, 245–254.
- McHenry, M.C.; Easley, K.A.; Locker, G.A. Vertebral osteomyelitis: Long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin. Infect Dis. 2002, 34, 1342–1350.
- Pigrau-Serrallach, C.; Rodriguez-Pardo, D. Bone and joint tuberculosis. Eur. Spine J. 2013, 22 (Suppl. 4), 556–566.
- Solis Garcia del Pozo, J.; Vives Soto, M.; Solera, J. Vertebral osteomyelitis: Long-term disability assessment and prognostic factors. J. Infect. 2007, 54, 129–134.
- Wang, D. Diagnosis of tuberculous vertebral osteomyelitis (TVO) in a developed country and literature review. Spinal Cord 2005, 43, 531–542.
- Kumar, R.; Srivastava, A.K.; Tiwari, R.K. Surgical management of Pott’s disease of the spine in pediatric patients: A single surgeon’s experience of 8 years in a tertiary care center. J. Pediatr. Neurosci. 2011, 6 (Suppl. 1), S101–S108.
- Tuli, S.M. Historical aspects of Pott’s disease (spinal tuberculosis) management. Eur. Spine J. 2013, 22, 529–538.
- Maron, R.; Levine, D.; Dobbs, T.E.; Geisler, W.M. Two cases of pott disease associated with bilateral psoas abscesses: Case report. Spine 2006, 31, E561–E564.
- Bruchfeld, J.; Correia-Neves, M.; Källenius, G. Tuberculosis and HIV Coinfection. Cold Spring Harb. Perspect. Med. 2015, 5, a017871.
- Tang, L.; Liu, S.; Bao, Y.C.; Gao, R.X.; Han, C.F.; Sun, X.C.; Zhang, W.L.; Feng, S.Q. Study on the relationship between vitamin D deficiency and susceptibility to spinal tuberculosis. Int. J. Surg. 2017, 44, 99–103.
- Rajasekaran, S.; Soundararajan, D.C.R.; Shetty, A.P.; Kanna, R.M. Spinal Tuberculosis: Current Concepts. Glob. Spine J. 2018, 8 (Suppl. 4), 96s–108s.
- Khoo, L.T.; Mikawa, K.; Fessler, R.G. A surgical revisitation of Pott distemper of the spine. Spine J. 2003, 3, 130–145.
- Gardini, G.; Gregori, N.; Matteelli, A.; Castelli, F. Mycobacterial skin infection. Curr. Opin. Infect. Dis. 2022, 35, 79–87.
- Rajasekaran, S.; Kanna, R.M.; Shetty, A.P. Pathophysiology and Treatment of Spinal Tuberculosis. JBJS Rev. 2014, 2, e4.
- Weaver, P.; Lifeso, R.M. The radiological diagnosis of tuberculosis of the adult spine. Skelet. Radiol. 1984, 12, 178–186.
- Jung, J.H.; Choi, S.; Kang, Y.; Cho, D.C.; Lee, S.M.; Park, T.I.; Choe, B.H.; Kim, D.; Kang, B. Development of Spinal Tuberculosis in an Adolescent With Crohn’s Disease After Infliximab Therapy: A Case Report With Literature Review. Front. Pediatr. 2021, 9, 802298.
- Kubihal, V.; Sharma, R.; Krishna Kumar, R.G.; Chandrashekhara, S.H.; Garg, R. Imaging update in spinal tuberculosis. J. Clin. Orthop. Trauma 2022, 25, 101742.
- Garg, B.; Mehta, N.; Mukherjee, R.N.; Swamy, A.M.; Siamwala, B.S.; Malik, G. Epidemiological Insights from 1,652 Patients with Spinal Tuberculosis Managed at a Single Center: A Retrospective Review of 5-Year Data. Asian Spine J. 2022, 16, 162–172.
- Jain, A.K.; Rajasekaran, S.; Jaggi, K.R.; Myneedu, V.P. Tuberculosis of the Spine. J. Bone Jt. Surg. Am. 2020, 102, 617–628.
- Nussbaum, E.S.; Rockswold, G.L.; Bergman, T.A.; Erickson, D.L.; Seljeskog, E.L. Spinal tuberculosis: A diagnostic and management challenge. J. Neurosurg. 1995, 83, 243–247.
This entry is offline, you can click here to edit this entry!
