Salt (NaCl) intake is processed by receptors in the tongue and digestive system, which transmit the information to the nucleus of the solitary tract via a neural pathway (chorda tympani/vagus nerves) and to circumventricular organs, including the subfornical organ and area postrema, via a humoral pathway (blood/cerebrospinal fluid). Circuits are formed that stimulate or inhibit homeostatic sodium (Na) intake involving participation of the parabrachial nucleus, pre-locus coeruleus, medial tuberomammillary nuclei, median eminence, paraventricular and supraoptic nuclei, and other structures with reward properties such as the bed nucleus of the stria terminalis, central amygdala, and ventral tegmental area. Finally, the kidney uses neural signals (e.g., renal sympathetic nerves) and vascular (e.g., renal perfusion pressure) and humoral (e.g., renin–angiotensin–aldosterone system, cardiac natriuretic peptides, antidiuretic hormone, and oxytocin) factors to promote Na excretion or retention and thereby maintain extracellular fluid volume.
- sodium homeostasis
- hypernatremia
- hyponatremia
1. Introduction
2. Behavioral Mechanism: Salt Intake
2.1. Detection and Processing in the Oral Cavity
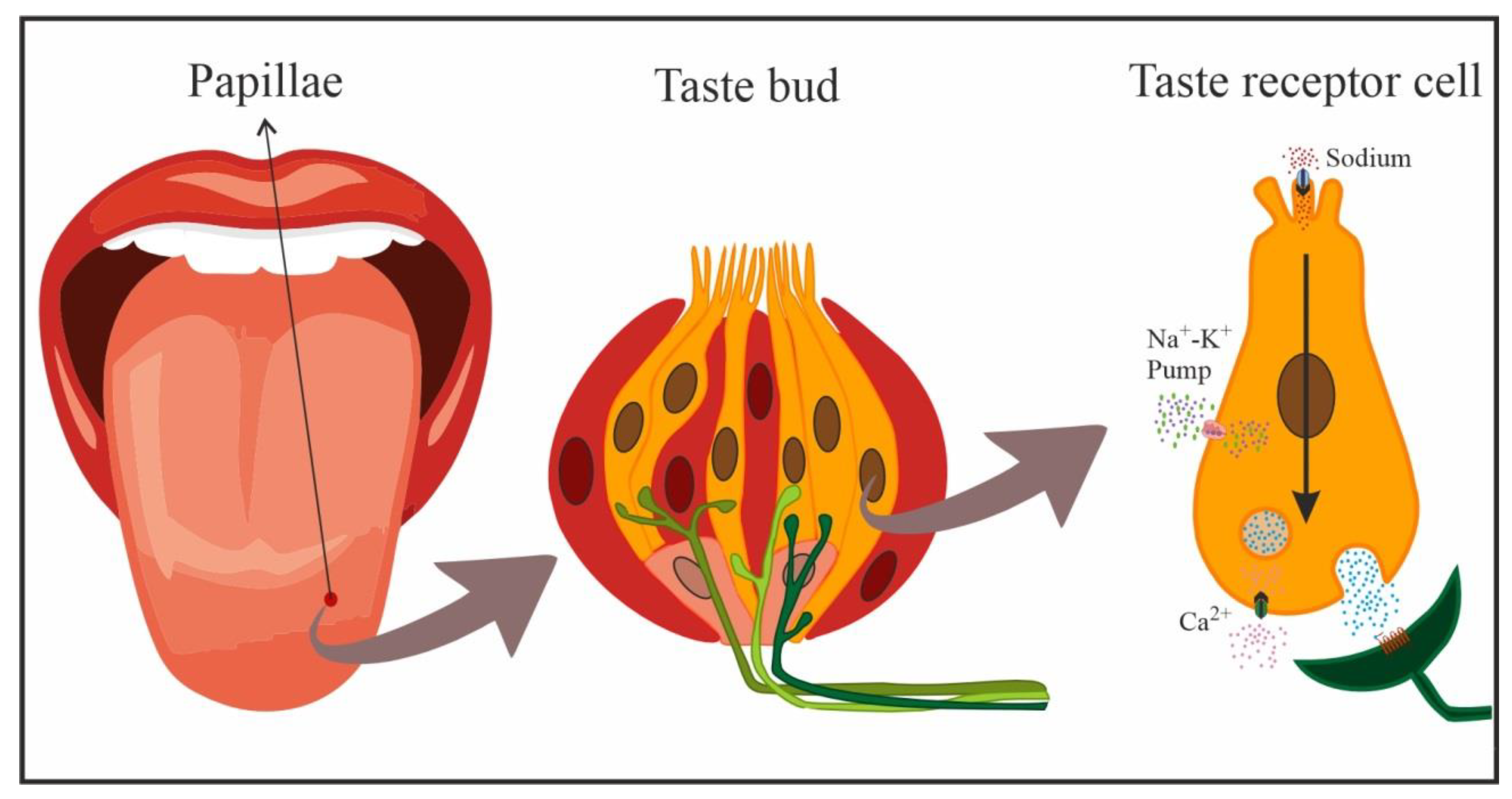
2.2. Sodium Detection and Processing in the Gastrointestinal System
This entry is adapted from the peer-reviewed paper 10.3390/nu15020395
References
- Fessler, D.M.T. An Evolutionary Explanation of the Plasticity of Salt Preferences: Prophylaxis against Sudden Dehydration. Med. Hypotheses 2003, 61, 412–415.
- Leshem, M. Salt Need Needs Investigation. Br. J. Nutr. 2020, 123, 1312–1320.
- Schulkin, J. Sodium Hunger: The Search for a Salty Taste; Cambridge University Press: Cambridge, UK, 1991; ISBN 978-0-521-35368-7.
- Bie, P. Mechanisms of Sodium Balance: Total Body Sodium, Surrogate Variables, and Renal Sodium Excretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R945–R962.
- Strazzullo, P.; Leclercq, C. Sodium. Adv. Nutr. 2014, 5, 188–190.
- Verbalis, J.G.; Stricker, E.M. Neuroendocrine Regulation of Fluid Intake and Homeostasis. In Neuroendocrinology in Physiology and Medicine; Conn, P.M., Freeman, M.E., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 317–334. ISBN 978-1-61737-153-0.
- Mahía, J.; Bernal, A. Animal Models for Diabetes Insipidus. Handb. Clin. Neurol. 2021, 181, 275–288.
- Noda, M.; Matsuda, T. Central Regulation of Body Fluid Homeostasis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2022, 98, 283–324.
- Lundy, R.F. Potential Mechanisms for Functional Changes in Taste Receptor Cells Following Sodium Deficiency in Mammals. Neurosci. Biobehav. Rev. 1998, 23, 103–109.
- McCaughey, S.A.; Scott, T.R. The Taste of Sodium. Neurosci. Biobehav. Rev. 1998, 22, 663–676.
- Bigiani, A. Does ENaC Work as Sodium Taste Receptor in Humans? Nutrients 2020, 12, 1195.
- Diepeveen, J.; Moerdijk-Poortvliet, T.C.W.; van der Leij, F.R. Molecular Insights into Human Taste Perception and Umami Tastants: A Review. J. Food Sci. 2022, 87, 1449–1465.
- Lindemann, B. Taste Reception. Physiol. Rev. 1996, 76, 719–766.
- Wu, A.; Dvoryanchikov, G.; Pereira, E.; Chaudhari, N.; Roper, S.D. Breadth of Tuning in Taste Afferent Neurons Varies with Stimulus Strength. Nat. Commun. 2015, 6, 8171.
- DeSimone, J.A.; Lyall, V. Taste Receptors in the Gastrointestinal Tract III. Salty and Sour Taste: Sensing of Sodium and Protons by the Tongue. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G1005–G1010.
- Miyamoto, T.; Fujiyama, R.; Okada, Y.; Sato, T. Acid and Salt Responses in Mouse Taste Cells. Prog. Neurobiol. 2000, 62, 135–157.
- Oakley, B.; Witt, M. Building Sensory Receptors on the Tongue. J. Neurocytol. 2004, 33, 631–646.
- Travers, J.B.; Travers, S.P.; Norgren, R. Gustatory Neural Processing in the Hindbrain. Annu. Rev. Neurosci. 1987, 10, 595–632.
- Kinnamon, S.C.; Finger, T.E. Recent Advances in Taste Transduction and Signaling. F1000Res 2019, 8, F1000 Faculty Rev-2117.
- Chandrashekar, J.; Kuhn, C.; Oka, Y.; Yarmolinsky, D.A.; Hummler, E.; Ryba, N.J.P.; Zuker, C.S. The Cells and Peripheral Representation of Sodium Taste in Mice. Nature 2010, 464, 297–301.
- Herness, M.S. Aldosterone Increases the Amiloride-Sensitivity of the Rat Gustatory Neural Response to NaCl. Comp. Biochem. Physiol. Comp. Physiol. 1992, 103, 269–273.
- Shigemura, N.; Iwata, S.; Yasumatsu, K.; Ohkuri, T.; Horio, N.; Sanematsu, K.; Yoshida, R.; Margolskee, R.F.; Ninomiya, Y. Angiotensin II Modulates Salty and Sweet Taste Sensitivities. J. Neurosci. 2013, 33, 6267–6277.
- Frank, M.E.; Contreras, R.J.; Hettinger, T.P. Nerve Fibers Sensitive to Ionic Taste Stimuli in Chorda Tympani of the Rat. J. Neurophysiol. 1983, 50, 941–960.
- McCaughey, S.A. Characterization of Mouse Chorda Tympani Responses Evoked by Stimulation of Anterior or Posterior Fungiform Taste Papillae. Neurosci. Res. 2019, 141, 43–51.
- Spector, A.C.; Grill, H.J. Salt Taste Discrimination after Bilateral Section of the Chorda Tympani or Glossopharyngeal Nerves. Am. J. Physiol. 1992, 263, R169–R176.
- Stricker, E.M.; Gannon, K.S.; Smith, J.C. Thirst and Salt Appetite Induced by Hypovolemia in Rats: Analysis of Drinking Behavior. Physiol. Behav. 1992, 51, 27–37.
- Altschuler, S.M.; Bao, X.; Bieger, D.; Hopkins, D.A.; Miselis, R.R. Viscerotopic Representation of the Upper Alimentary Tract in the Rat: Sensory Ganglia and Nuclei of the Solitary and Spinal Trigeminal Tracts. J. Comp. Neurol. 1989, 283, 248–268.
- Hamilton, R.B.; Norgren, R. Central Projections of Gustatory Nerves in the Rat. J. Comp. Neurol. 1984, 222, 560–577.
- Arnedo, M.; Gallo, M.; Agüero, A.; Puerto, A. Effects of Medullary Afferent Vagal Axotomy and Area Postrema Lesions on Short-Term and Long-Term NaCl-Induced Taste Aversion Learning. Physiol. Behav. 1990, 47, 1067–1074.
- Burman, A.; Kaji, I. Luminal Chemosensory Cells in the Small Intestine. Nutrients 2021, 13, 3712.
- Sbarbati, A.; Osculati, F. The Taste Cell-Related Diffuse Chemosensory System. Prog. Neurobiol. 2005, 75, 295–307.
- Zafra, M.A.; Prados, M.; Molina, F.; Puerto, A. Capsaicin-Sensitive Afferent Vagal Fibers Are Involved in Concurrent Taste Aversion Learning. Neurobiol. Learn. Mem. 2006, 86, 349–352.
- Zimmerman, C.A.; Huey, E.L.; Ahn, J.S.; Beutler, L.R.; Tan, C.L.; Kosar, S.; Bai, L.; Chen, Y.; Corpuz, T.V.; Madisen, L.; et al. A Gut-to-Brain Signal of Fluid Osmolarity Controls Thirst Satiation. Nature 2019, 568, 98–102.
- Johnson, A.K.; Thunhorst, R.L. The Neuroendocrinology of Thirst and Salt Appetite: Visceral Sensory Signals and Mechanisms of Central Integration. Front. Neuroendocr. 1997, 18, 292–353.
- Mediavilla, C.; Bernal, A.; Mahía, J.; Puerto, A. Nucleus of the Solitary Tract and Flavor Aversion Learning: Relevance in Concurrent but Not Sequential Behavioral Test. Behav. Brain Res. 2011, 223, 287–292.
- Mediavilla, C.; Molina, F.; Puerto, A. Concurrent Conditioned Taste Aversion: A Learning Mechanism Based on Rapid Neural versus Flexible Humoral Processing of Visceral Noxious Substances. Neurosci. Biobehav. Rev. 2005, 29, 1107–1118.
- Contreras, R.J.; Kosten, T. Changes in Salt Intake after Abdominal Vagotomy: Evidence for Hepatic Sodium Receptors. Physiol. Behav. 1981, 26, 575–582.
- Chernigovsky, V.N. The Significance of Interoceptive Signals in the Food Behavior in Animals. In The Internal Environment and Alimentary Behavior; Brazier, M.A.B., Ed.; Brain and Behavior; American Institute of Biological Sciences, University of California: Los Angeles, CA, USA, 1963; pp. 319–349.
- Blackshaw, L.A.; Grundy, D. Effects of 5-Hydroxytryptamine on Discharge of Vagal Mucosal Afferent Fibres from the Upper Gastrointestinal Tract of the Ferret. J. Auton. Nerv. Syst. 1993, 45, 41–50.
- Mei, N. Intestinal Chemosensitivity. Physiol. Rev. 1985, 65, 211–237.
- Mei, N.; Garnier, L. Osmosensitive Vagal Receptors in the Small Intestine of the Cat. J. Auton. Nerv. Syst. 1986, 16, 159–170.
- Zhu, J.X.; Wu, X.Y.; Owyang, C.; Li, Y. Intestinal Serotonin Acts as a Paracrine Substance to Mediate Vagal Signal Transmission Evoked by Luminal Factors in the Rat. J. Physiol. 2001, 530, 431–442.
- Kahrilas, P.J.; Rogers, R.C. Rat Brainstem Neurons Responsive to Changes in Portal Blood Sodium Concentration. Am. J. Physiol. 1984, 247, R792–R799.
- Morita, H.; Yamashita, Y.; Nishida, Y.; Tokuda, M.; Hatase, O.; Hosomi, H. Fos Induction in Rat Brain Neurons after Stimulation of the Hepatoportal Na-Sensitive Mechanism. Am. J. Physiol. 1997, 272, R913–R923.
- Barraco, R.; El-Ridi, M.; Ergene, E.; Parizon, M.; Bradley, D. An Atlas of the Rat Subpostremal Nucleus Tractus Solitarius. Brain Res. Bull. 1992, 29, 703–765.
- Herbert, H.; Moga, M.M.; Saper, C.B. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 1990, 293, 540–580.
- Hall, J.E.; Guyton, A.C.; Hall, M.E. Tratado de Fisiología Médica; 14th ed.; Elsevier: Barcelona, Spain, 2021; ISBN 978-84-13-82013-2.
- Linz, B.; Saljic, A.; Hohl, M.; Gawałko, M.; Jespersen, T.; Sanders, P.; Böhm, M.; Linz, D. Inhibition of Sodium-Proton-Exchanger Subtype 3-Mediated Sodium Absorption in the Gut: A New Antihypertensive Concept. IJC Heart Vasc. 2020, 29, 100591.
- Spiller, R.C. Intestinal Absorptive Function. Gut 1994, 35, S5–S9.
- Binder, H.J. Movimiento de Fluidos y Electrolitos Intestinales—Boron y Boulpaep.Manual de Fisiología Médica. In Boron & Boulpaep Concise Medical Physiology; Boron, W.F., Boulpaep, E.L., Eds.; Elsevier: Philadelphia, PA, USA, 2021; pp. 476–484. ISBN 978-0-323-65530-9.
- Negussie, A.B.; Dell, A.C.; Davis, B.A.; Geibel, J.P. Colonic Fluid and Electrolyte Transport 2022: An Update. Cells 2022, 11, 1712.
- Afsar, B.; Vaziri, N.D.; Aslan, G.; Tarim, K.; Kanbay, M. Gut Hormones and Gut Microbiota: Implications for Kidney Function and Hypertension. J. Am. Soc. Hypertens. 2016, 10, 954–961.
- Coric, T.; Hernandez, N.; de la Rosa, D.A.; Shao, D.; Wang, T.; Canessa, C.M. Expression of ENaC and Serum- and Glucocorticoid-Induced Kinase 1 in the Rat Intestinal Epithelium. Am. J. Physiol. Liver Physiol. 2004, 286, G663–G670.
- Musch, M.W.; Lucioni, A.; Chang, E.B. Aldosterone Regulation of Intestinal Na Absorption Involves SGK-Mediated Changes in NHE3 and Na+ Pump Activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G909–G919.
- Ch’ng, S.S.; Lawrence, A.J. The Subfornical Organ in Sodium Appetite: Recent Insights. Neuropharmacology 2019, 154, 107–113.
- Sladek, C.D.; Armstrong, W.E. Osmotic Control of Vasopressin Release. Trends Neurosci. 1985, 8, 166–169.
- Hiyama, T.Y.; Noda, M. Sodium Sensing in the Subfornical Organ and Body-Fluid Homeostasis. Neurosci. Res. 2016, 113, 1–11.
- Miller, R.L.; Wang, M.H.; Gray, P.A.; Salkoff, L.B.; Loewy, A.D. ENaC-Expressing Neurons in the Sensory Circumventricular Organs Become c-Fos Activated Following Systemic Sodium Changes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1141–R1152.
- Solár, P.; Zamani, A.; Kubíčková, L.; Dubový, P.; Joukal, M. Choroid Plexus and the Blood-Cerebrospinal Fluid Barrier in Disease. Fluids Barriers CNS 2020, 17, 35.
- Wang, S.; Liu, J.; Cai, H.; Liu, K.; He, Y.; Liu, S.; Guo, Y.; Guo, L. High Salt Diet Elevates the Mean Arterial Pressure of SLC14α1 Gene Depletion Mice. Life Sci. 2020, 254, 117751.
- Peruzzo, M.; Milani, G.P.; Garzoni, L.; Longoni, L.; Simonetti, G.D.; Bettinelli, A.; Fossali, E.F.; Bianchetti, M.G. Body Fluids and Salt Metabolism—Part II. Ital. J. Pediatr. 2010, 36, 78.
