Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Contrary to the biosynthetic pathways of many terpenoids, which are well characterized and elucidated, their transport inside subcellular compartments and the secretion of reaction intermediates and final products at the short- (cell-to-cell), medium- (tissue-to-tissue), and long-distance (organ-to-organ) levels are still poorly understood, with some limited exceptions.
- terpenoids
- carotenoids
- apocarotenoids
- ABA
- strigolactones
1. Introduction
Plant specialized metabolites (PSMs), previously referred as secondary metabolites, are small molecules (<2000 Da) synthesized and accumulated in specific tissues and developmental stages and/or under particular environmental conditions, which own different functions aiming at generally improving plant fitness, including physiological aspects and responses to biotic and abiotic stresses [1]. These metabolites are generally produced in specific organs, cells, or subcellular compartments that are tightly subjected to intra- or inter-cellular transport through biological membranes. Several transport mechanisms have been described and characterized in past years for many classes of PSMs, such as alkaloids, phenylpropanoids, and terpenoids. Among these classes of compounds, terpenoids, also referred as isoprenoids, are the most diverse, with about 80,000 distinct compounds that have important implications in plant growth, development, and adaptation, and in interaction with other organisms [2,3,4]. Relatively few isoprenoids have essential roles in plant development and fitness, such as carotenoids, sterols, gibberellins, chlorophylls, and plastoquinones [2,3,5]. The vast majority of plant terpenoids are PSMs involved in plant–environment interactions [6,7]. For example, diverse terpenoids, including carotenoids, their derivatives, and volatile terpenoids, have indispensable functions in attracting pollinators and seed dispersers [3,8,9], while others have roles in mediating beneficial symbiotic relationships that improve plant fitness [10]. Terpenoids also take part in allelopathic interactions and in abiotic stress responses [11,12]. Many terpenoids are instead potent toxic compounds that serve as chemical defenses against herbivores, insects, and microbial pathogens [2,3,13]. Together with the expansion in terpenoid metabolism, which takes place when land plants face environmental changes and biotic or abiotic stresses [3,5], several mechanisms to improve their differential compartmentation, secretion, and bioactivity have been evolved.
2. General Transport Mechanisms for PSMs
The transport of molecules between and within cells generally follows different types of mechanisms, based on the nature of the compound. In more detail, gasses and some small lipophilic compounds, including a few volatiles, can pass through membranes by simple diffusion [17], while charged or bigger molecules, such as metals, ions, sugars, vitamins, volatiles, and general/specialized metabolites, need a protein- or lipid-mediated translocation or vesicle-mediated transport [14,18,19,20,21].
Within cells, the intracellular trafficking of vesicles that bud off from the endoplasmic reticulum (ER) allows the secretion of proteins, lipids, components of the membranes, and other molecules into the plasma membrane and apoplast, or to subcellular compartments, such as vacuole and peroxisomes [18,22,23,24]. The transport is basically obtained by the fusion of vesicles to cellular membranes, with the release of their content. Besides vesicle-mediated transport, the main mechanism responsible for PSMs is protein-mediated transport across membranes [25,26]. The transmembrane transporters generally involved in this process belong to five families: (i) ATP-binding cassette (ABC) transporters, (ii) multidrug and toxin extrusion (MATE) proteins, (iii) nitrate-peptide transporter (NPF), (iv) purine uptake permease (PUP), and (v) AWPM-19 family proteins [14,25,27]. Among them, only ABC transporters mediate the primary active transport (e.g., the transport is driven by the hydrolysis energy of ATP), while the others mediate the secondary active transport (transport driven by an electrochemical gradient). Overall, the different transporter classes are described as follows:
-
ABC transporters function as ATP-driven pumps to import and export molecules of diverse types, such as metals, xenobiotics, hormones, pigments, and largely diversified specialized metabolites [16,28]. The ABC family is highly expanded in the plant kingdom in comparison to other organisms, and in Arabidopsis thaliana many members have been functionally characterized [15]. This expansion is mainly due to the fact that plants are sessile organisms that need to adapt to environmental changes and modulate the expression of genes involved in the synthesis, storage, and release of specific metabolites that serve to cope with the modified conditions [29]. In general, nine subfamilies of ABC transporters have been identified in plants (from ABCA to ABCI, with the exception of the ABCH proteins that have not been found yet) [30], with the B, C, and G subfamilies being the most abundant. The members of these subfamilies have been described as being involved in the translocation of PSMs, toxic compounds, and phytohormones [15], molecules that are particularly important for the selective advantage of plants to terrestrial life during evolution [31]. ABCGs and ABCBs are plasma membrane-localized ABC transporters responsible for the translocation of PSMs between cells, while ABCCs are generally present in the tonoplast, where they contribute to the vacuolar accumulation of metabolites. More particularly, the ABCG subfamily, also called pleiotropic drug resistance (PDR) transporters, is one of the predominant class of transporters involved in important biological functions, such as pathogen defense, abiotic stress tolerance, cuticular formation, and phytohormone transport [14,15,32]. For a detailed and updated status of ABC transporter proteins in plants, see [16].
-
MATEs are secondary transporters that use proton or sodium ion gradients to translocate substrates across membranes [33,34]. Like ABC transporters, MATEs have been subjected to a high expansion through tandem and segmental duplication events during the course of evolution. In Arabidopsis, 58 members of MATE transporters, also called DETOXIFICATION (DTX) proteins, have been identified [34]. MATEs exhibit a wide array of functions in plants, including detoxification of xenobiotics and transport of metals, phytohormones, and PSMs [14,25]. Four main classes of MATEs have been identified to date [33]; many MATE members are located in the plasma membrane and function as exporters, but several tonoplast members have also been identified and described as transporters for the vacuolar sequestration of PSMs (especially alkaloids and phenylpropanoids) [21,35,36,37].
-
NPFs, previously known as NRTs, use proton gradient to transport different classes of compounds such as nitrate, amino acids, peptides, phytohormones, and PSMs [25]. After the first discovery of a NPF as a nitrate transporter in A. thaliana [38], NPFs were found to translocate amino acids and peptides [39,40]. Lately, it was demonstrated that NPFs are responsible for the transport of glucosinolates and alkaloid derivatives [41,42,43,44,45,46]. To date, eight subfamilies of NPFs have been identified [43] based on the nomenclature proposed in [47].
-
For several years, PUP transporters were thought to be involved in the translocation of purine nucleobase substrates. However, in 2011, it was demonstrated that a Nicotiana tabacum PUP-like homolog, named NUP1, is involved in the transport of alkaloid nicotine across the plasma membrane [48]. These findings pave the way for the study of PUP-like proteins as PSM transporters. Two other studies displayed that PUP-like proteins are involved in the transport of a few other alkaloids [49,50]. Interestingly, the PUP-like family originated during terrestrial plant evolution between the bryophytes and the lycophytes. A phylogenetic study showed an extensive pattern of gene duplication and diversification within the angiosperm lineage, with sub-functionalization and neo-functionalization of PUP-like transporters [51].
-
More recently, a new class of transporters, belonging to the AWPM-19 family, has been identified in wheat and rice, albeit they have been also described in Arabidopsis [27,52,53]. AWPM-19 transporter proteins are encoded by an ancient, highly conserved member of the plasma membrane 4 gene family, and they have been found to play a role in abscisic acid (ABA) transport. Actually, in species, such as rice, in which no authentic ABC-type ABA transporters have been identified, the OsPM1 AWPM-19 member has been considered the main transporter responsible for ABA import and drought-related responses [27].
Altogether, of these classes, the most well characterized for the translocation of PSMs are ABC- and MATE-type families.
Besides these classes of transporters, other transmembrane protein families have been described to have a role in hormone translocation and may be discovered in the future to be involved in the transport of other classes of PSMs.
For instance, Sugars Will Eventually be Exported Transporters (SWEETs) are responsible for both the uptake and efflux of sugars in plant cells [54,55,56]. SWEETs are important uniporters for intracellular and intercellular sugar translocation that are strongly induced upon pathogen invasion [57]. The transport is driven by the sugar gradient, and SWEETs localize to different compartments, mainly in the plasma membrane, but also in the tonoplast and the Golgi membranes (see [55] for more details). Surprisingly, SWEET proteins can also be involved in hormone transport. This discovery has expanded the knowledge on this class of proteins, which can be considered as a broader range of transporters [55].
Another class of transmembrane transporters participating in the transport of phytohormones are PIN-FORMED (PIN) proteins, which are secondary transporters involved in the export of auxins [58,59,60]). The majority of PIN proteins localize to the plasma membrane and transport auxins from the cytosol to the apoplast; other PINs localize to the ER and are responsible for auxin transport from the cytosol to the ER lumen, thus regulating auxin homeostasis [58].
In addition to the translocation mechanisms mentioned above, lipid-transfer proteins (LTPs), which are small proteins of maximum 10 kDa, can play a role in facilitating the diffusion of hydrophobic molecules through membranes or can assist transmembrane transporters, such as ABCGs [61]. In more detail, LTPs can diffuse through membranes and present a tunnel-like hydrophobic cavity, which binds lipids or other hydrophobic compounds that are deposited in the apoplast, such as lignins or suberin [61,62,63]. However, since it has been shown that members of this family are able to bind isoprenoids (e.g., carotenoids) [64,65,66], it cannot be excluded that these proteins might also be involved in the translocation of lipophilic PSMs.
3. Function and Transport of Terpenes
3.1. Generalities
Terpenes constitute a large and structurally diverse family of primary and specialized metabolites in plants [2,5]. Some terpenes are produced in low amounts and serve as plant phytohormones, such as gibberellins (GAs), cytokinins (CKs), abscisic acid (ABA), and strigolactones (SLs); others, such as carotenoids, are produced in bulk amounts and have a role as light-harvesting pigments and photoprotectors in photosynthesis, or an ecological role as pigments to attract pollinators and seed dispersers. In addition, plants contain a huge variety of monoterpenes, sesquiterpenes, diterpenes, triterpenes, and carotenoid derivatives (apocarotenoids) that function as specialized metabolites with important ecological functions in the interaction of plants with other organisms [6,7,67,68,69]. Terpenes have simple hydrocarbon structures, while terpenoids present different functional groups [2]. Numerous terpenoids are bioactive molecules that are used in medicine (i.e., taxol or artemisinin) or as flavor and fragrance compounds [70,71,72]; therefore, there is a great interest on the elucidation and engineering of the biosynthetic pathways involved in their transport within or between cells.
3.2. Biosynthesis and Functions
Terpenes can be classified, based on the number of the five-carbon isoprene units, as hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterpenes (C25), triterpenes (C30), and tetraterpenes (C40). They are all synthesized from the condensation of the five-carbon isoprenoid precursors, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). IPP and DMAPP are generated by two independent, cell-compartmentalized biosynthesis: the cytosolic mevalonic acid (MVA) pathway, and the plastidial methylerythritol phosphate (MEP) pathway [5]. In the MVA pathway, the precursors derived from acetyl-CoA are condensed by farnesyl diphosphate synthase (FPS) to form farnesyl diphosphate (FPP, C15), which is the precursor of sesquiterpenes, triterpenes, and sterols. On the contrary, in the MEP pathway, IPP and DMAPP are originated from pyruvate and glyceraldehyde-3-phosphate and are condensed by geranyl diphosphate synthase (GPS) to form geranyl diphosphate (GPP, C10), which is the direct precursor of monoterpenes, and by geranylgeranyl diphosphate synthase (GGPPS) to form geranylgeranyl diphosphate (GGPP, C20), which acts as a precursor for diterpenes and carotenoids [5,73].
After the formation of the precursors, FPP, GPP, and GGPP, the different terpenes are generated through the action of terpene synthases (TPS), which are enzymes implicated in the formation of primary terpene skeletons, and are then further modified by the action of various enzyme classes, such as cytochrome P450 hydroxylases, dehydrogenases, and glycosyl- and methyl-transferases [6,73]. These enzymes have various subcellular localization: for instance, P450 enzymes are generally located in the ER membrane [74], while other enzymes are placed essentially in the cytosol or in the ER surface [75,76,77,78]. Differently from terpenes that are synthesized in the cytosol, such as tri- and sesquiterpenes, the biosynthesis of diterpenes and tetraterpenes (and their derivatives) implies the translocation of intermediates from the plastid to the cytosol and/or ER, where the modification enzymes reside. This kind of transport is poorly understood, and several pieces are missing in a puzzle which is still being solved (Figure 1). The final products of terpenoid biosynthesis are then (i) accumulated in the cytosol, (ii) stored in the vacuole, or (iii) secreted into the apoplast. Generally, monoterpenes and sesquiterpenes are secreted through the plasma membrane and accumulate in the apoplast or in specialized structures, such as oil gland or glandular trichomes [79,80]. These molecules have strong ecological roles as phytochemicals against pathogens and herbivore attacks [81,82] or as volatiles emitted for the attraction of pollinators and natural enemies of herbivores [83,84]. Additionally, they are the main constituents of essential oils and are of great interest for their biological properties, such as antioxidant, anticancer, anti-inflammatory, antimicrobial, antiviral, anthelminthic, antinociceptive properties [70,71,72,85,86,87]. Terpenoids also contribute to the formation of complex molecules, such as monoterpene indole alkaloids (MIAs), which are PSMs with powerful pharmacological activities (e.g., the well-known anti-tumor agents vinblastine and vincristine) [88,89]. Among sesquiterpenes, a well-known member is β-caryophyllene, a volatile compound present in many essential oils, especially from clove, rosemary, and Cannabis sativa, that plays an important role in the defense against microbial pathogens [90,91]. Triterpenes are the largest subgroup of structurally diverse terpene molecules that includes sterols, steroids, and saponins. Triterpenoids derived from squalene [92] and its members have fundamental roles as wax and resin components, e.g., lupeol and β-amyrin, which are also the precursors of pentacyclic metabolites with distinct bioactivity against plant biotic stressors, such as betulinic acid (from the bark of birch tree, Betula pubescens) or glycyrrhizin (found in the roots and stolons of licorice, Glycyrrhiza glabra), which are also characterized by their pharmaceutical and, limited to the latter, sweetener properties [93,94]. Diterpenes also have important functions as biotic and abiotic interactions, and some of them act as phytohormones (for example, gibberellins or GAs [78]). Finally, carotenoids are a large group of tetraterpene pigments, with more than 600 different structures [7,9,95]. They are produced in plastids and are the precursors of apocarotenoids, which are enzymatic or non-enzymatic cleavage products that are subjected to several modifications, stored in the vacuoles or secreted outside the cells, and play a role as signal molecules, pigments, aromas, or hormones [96,97,98,99,100].
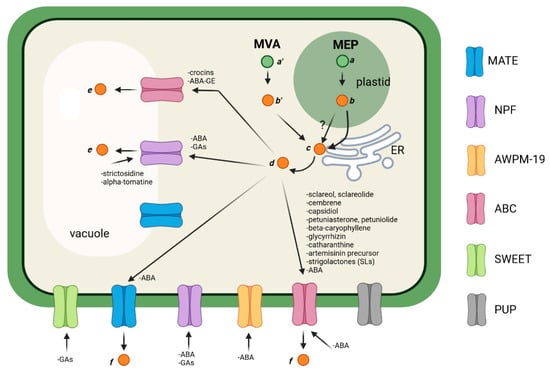
Figure 1. General scheme depicting the transport of terpenoids in plant cells.
Lipophilic terpenes can passively diffuse through membranes [101,102,103], or they are transported by sequestration in vesicles or lipid droplets that fuse with the membrane, by carrier proteins, such as LTP proteins, or through specific transmembrane transporters [61,104]. Differently, hydrophilic compounds cannot pass through membranes and are generally stored in the vacuole as conjugated (glucosylated or acylated) compounds [45] by the action of ABC or MATE transporters [45], or through the fusion of ER–derived vesicles [105,106]. The addition of sugars to terpene aglycones occurs later in the biosynthetic pathway, and it represents a mechanism to increase their stability, polarity, and water solubility, and to decrease their biological activity and toxicity [80,107].
3.3. Transport of C10-C15-C20-C30 Terpenoids (Mono-, Sesqui-, Di-, and Triterpenoids)
Although small volatile terpenes emitted by leaves, such as isoprene, are released by simple diffusion across biological membranes [108,109,110], bigger terpenes, including monoterpenes, generally require the presence of active transporters or vesicle-mediated transport [20,111]. For years, it was believed that volatile organic compounds (VOCs) cross membranes by diffusion; however, it was then demonstrated that lipophilic small compounds tend to accumulate into biological bilayers with detrimental effects on membrane integrity, demonstrating that different translocation mechanisms are involved [20,111]; for instance, in Petunia hybrida, an ABC transporter is responsible for the translocation of different VOCs across the plasma membrane [111]. Regarding terpenoids, in Vitis vinifera, different sesquiterpenoid volatiles are emitted in the flowers, and it has been demonstrated that the sesquiterpene synthase, valencene synthase (VvValCS), which is responsible for their synthesis, is localized to the outer edges of lipid vesicles in pollen grains [112]. Thus, this finding suggests that valencene is stored and secreted through lipid vesicles. Another study provided clues about the involvement of a vesicle-mediated transport of monoterpenes in the secretory cells of the glandular trichomes of Prostanthera ovalifolia. Indeed, the electron microscopy images show that plastids (where the biosynthesis of the precursor of monoterpenes takes place) are surrounded by vesicles that then fuse with the plasma membrane [113]. An additional work evidenced that a vesicle-mediated transport from the ER to the plasma membrane is involved in the subcellular movement of the sesquiterpenes copaene and β-caryophyllene in Sauromatum guttatum flowers [114]. β-Caryophyllene is a potential toxic volatile since, in the cytosol, it can react with proteins, leading to the formation of caryophyllene oxide (CPO) [115]. CPO induces increased reactive oxygen species (ROS) generation from mitochondria, leading to the induction of degenerative processes [112]. For this reason, the cells need to emit it in the headspace of the plant, and, here, it serves as a defense against bacterial pathogens that invade tissues [90,91]. Interestingly, for this compound, in addition to the vesicle-mediated transport, the involvement of an active transporter belonging to the ABCG subfamily has been demonstrated [81]. Plasma membrane-localized ABCGs are, in fact, the main class of transporters implicated in the secretion of terpenes [15,116]. For example, it has been shown that the exporter AaABCG3/AaPDR3 is responsible for β-caryophyllene secretion in Artemisia annua T-shaped trichomes and roots [81]. Of note, this transporter has high homology sequence with Nicotiana plumbaginifolia NpPDR1, N. tabacum NtPDR1, Arabidopsis thaliana AtPDR12, and Spirodela polyrrhiza SpTUR2 transporters [81]. The NpPDR1 of N. plumbaginifolia transports the antifungal diterpenes, sclareol and sclareolide, across the plasma membrane, resulting in their secretion on the glandular trichomes, where they function as antifungal compounds [117,118]. In contrast, the homolog of this transporter in N. tabacum, NtPDR1, is able to translocate not only sclareol, but also the diterpene cembrene [119] and the sesquiterpene capsidiol [120], a phytoalexin involved in the defense against pathogens. Additionally, the NbABCG1 and NbABCG2 transporters of N. benthamiana seem to be involved in the same transport [121,122], and an ortholog of the NpABC1 transporter has also been found in Arabidopsis, AtPDR12 [123] and S. polyrhiza, SpTUR2 [124]. Notably, SpTUR2 was the first plant PDR transporter to be characterized [125], and its expression in Arabidopsis plants leads to the acquisition of resistance to sclareol [124]. In P. hybrida, for instance, it was shown that the PhPDR2 transporter is involved in the defense against herbivores, secreting two steroidal-derived compounds (petuniasterone and petuniolide) [126]. Another well-known example is artemisinin, a potent anti-malarial sesquiterpene lactone that accumulates in the glandular trichomes of Artemisia annua [127]. Indeed, Wang and coauthors [128] demonstrated that two transporters from A. annua, the AaPDR2 transporter and the lipid-transfer protein 3 (AaLTP3), once expressed in N. benthamiana leaves, led to the accumulation of the precursor of artemisinin, (DH)AA (dihydro)artemisinic acid [DHAA]), that is then photochemically converted to artemisinin in the apoplastic space, suggesting the involvement of these transporters in vivo. Finally, a recent study reported the identification of four members of plasma membrane-localized ABCG transporters in Salvia miltiorrhiza Bunge, a plant used in traditional Chinese medicine to treat cardiovascular and cerebrovascular diseases; these transporters could be potentially involved in the export of tanshinone (a lipophilic diterpene) and salvianolic acid (a hydrophilic phenolic compound), which are metabolites highly accumulated in the roots and rhizomes of S. miltiorrhiza [129]. Additionally, in the present Special Issue, Kato and coauthors described the transport of the triterpene saponin glycyrrhizin in Glycyrrhiza glabra (licorice plant) by a H+-symporter in the plasma membrane and by an ATP-binding cassette transporter in the vacuole (with a high specificity for the aglycone form) [130].
An additional example of the vacuolar transport of terpenoids is represented by avenacin A-1 and triterpene saponins of oat [131]. Avenacin A-1 is a glucosylated antifungal compound that is stored in the vacuole, and its transport has not yet been elucidated, albeit the co-authors suggested that an ABC transporter might be the main responsible.
Some information is also available for the transport of MIAs. The majority of MIAs are derived from the assembly of tryptamine and the monoterpene secologanin to form the central intermediate strictosidine. The transporter responsible for the vacuolar export of this intermediate was identified in Catharanthus roseus: a NPF transporter, named CrNPF2.9, localizes to the tonoplast of leaf epidermal cells and translocates strictosidine from the vacuole to the cytosol [44]. Similarly, a Solanum lycopersicum NPF transporter called GORKY is responsible for the export of the steroidal glycoalkaloid α-tomatine and its derivatives from the vacuole to the cytosol [46]. Another characterized transporter involved in MIA translocation belongs to the ABC Family, G group. The plasma membrane CrTPT2 transporter from C. roseus is involved in the export of catharanthine to the leaf surface [132].
Regarding diterpene transport, different studies have shed light on the transport mechanisms of the hormone gibberellin (GA). GA biosynthesis starts in plastids, with the formation of the intermediate Ent-kaurene, then proceeds in the ER, and finishes in the cytosol, where different GAs are formed [78]. The mechanisms responsible for the release of the precursors from the plastid and the ER have not yet been identified, while much information regarding the GA movement across the plasma membrane is available. Indeed, GA moves between cells through different mechanisms, with the ion trap being the main mechanism that allows GAs to enter cells [78]. Following entrance into the cytosol, GAs are converted into their charged forms (due to the weak alkaline pH of 7.2), which prevents their further simple diffusion; thus, the export of GAs requires the activity of specific transporters [133]. While GA efflux transporters have not yet been identified, several influx transporters have been described [134]: it was demonstrated in vitro in yeast cells that different NPF transporters are able to transport diverse GAs, as well as ABA and other hormones, such as jasmonic acid (JA) [135,136]. In more detail, these studies showed that, of the 45 NPF transporters identified, several are able to import different types of GAs [136,137]. Another type of transporter that has been revealed to be involved in the influx of GAs belongs to the class of SWEET transporters. In particular, the A. thaliana AtSWEET13 and AtSWEET14 transporters have been shown to be able to transport GAs in different forms (active and non-active) [138]. In addition, in rice, a SWEET transporter, OsSWEET3a, was found to be involved in the transport of GAs [139]. Interestingly, GA transport by SWEET proteins evolved independently during plant evolution: in fact, in Arabidopsis, it arose from sucrose SWEET transporters, while in rice, it arose from glucose SWEETs [139]. GAs are also accumulated in the vacuole, especially as a reservoir to use during the suberization process. It was demonstrated that GAs, as well as ABA, are loaded into the pericycle vacuoles at the phloem in the roots of A. thaliana to form a storage pool, which is later released into the endodermis to induce suberization [140]. An NPF transporter, named NPF2.14, is a tonoplast-localized transporter in pericycle cells able to transport GAs and ABA from the cytosol to the vacuole. When the root elongates and GAs and ABA levels in the vacuole become high, the hormones are exported out of the pericycle vacuole and imported into the endodermis by the NPF3.1 transporter [140].
This entry is adapted from the peer-reviewed paper 10.3390/plants12030634
This entry is offline, you can click here to edit this entry!
