Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Macromolecules containing acidic fragments in side-groups—polyacids—occupy a special place among synthetic polymers. Properties and applications of polyacids are directly related to the chemical structure of macromolecules: the nature of the acidic groups, polymer backbone, and spacers between the main chain and acidic groups. The chemical nature of the phosphorus results in the diversity of acidic >P(O)OH fragments in sidechain phosphorus-containing polyacids (PCPAs) that can be derivatives of phosphoric or phosphinic acids.
- biocompatibility
- coordination polymerization
- free-radical polymerization
- metathesis polymerization
- phosphonic acids
- polyphosphoesters
1. Introduction
Polymers containing multiple acidic (–C(O)OH, S(O)2OH, –P(O)(OH)2, etc.) fragments distributed throughout the polymer backbone—polyacids, more commonly named anionic polyelectrolytes—constantly attract the researchers’ attention [1][2][3]. Enhanced hydrophilicity, proton conductivity, ability to complexation with metal ions and organic bases, and biocompatibility are occasionally shown by this type of polymer—this is not a complete list of attractive properties of polyacids that determine a variety of their applications. Phosphorus-containing polyacids (PCPAs) represent a separate group of polyanionic electrolytes due to the relatively high acidity of the –P(O)(OH)2 group, higher biocompatibility, and bone and mineral affinity of phosphates.
2. Design and Synthesis of Sidechain PCPAs
2.1. Synthetic Approaches to Sidechain PCPAs: An Overview
Most of the synthetic approaches to sidechain PCPAs use (co)polymerization of the phosphorus-containing vinyl monomers (Scheme 1a,b). The alternative approach is based on the post-modification of the (co)polymers (Scheme 1c).
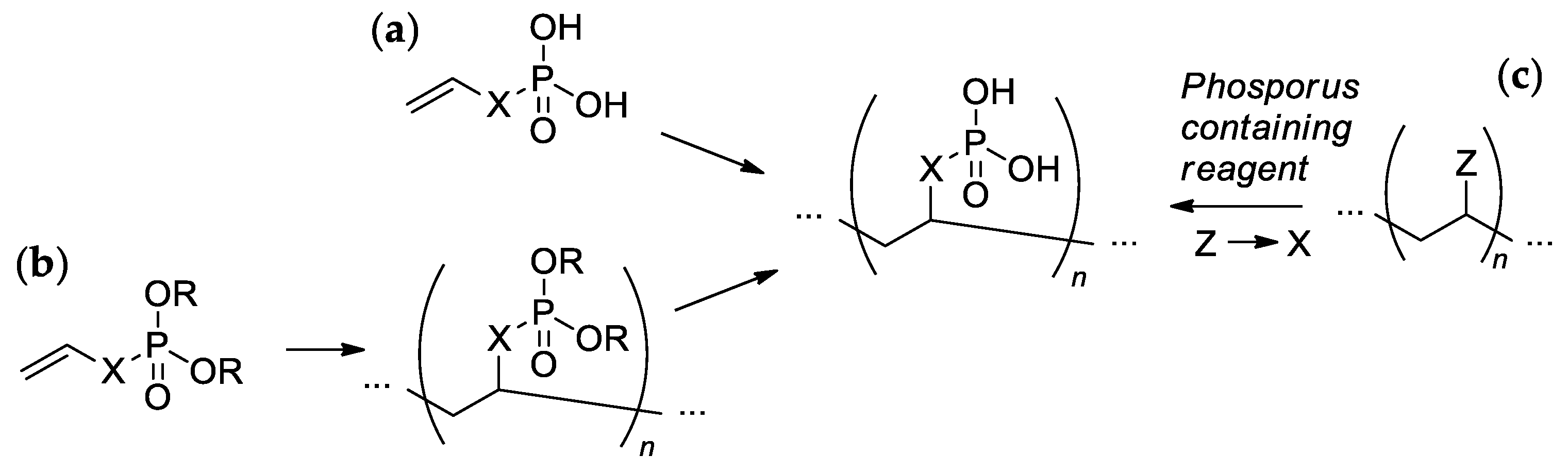
Scheme 1. The common synthetic approaches to sidechain PCPAs: (a) polymerization of unsaturated phosphoric or phosphinic acids; (b) polymerization of unsaturated phosphoesters followed by hydrolysis; (c) phosphorylation of polymers.
2.2. Phosphorus Containing Vinyl Monomers
Vinyl monomers, containing phosphonate or phosphate fragments, are definitely starting compounds suitable for the synthesis of sidechain PCPAs, and that was the reason why researchers chose to devote a separate section to these compounds. The synthesis and chemistry of the phosphorus-containing vinyl monomers were discussed previously in the review of Macarie and Ilia [4], outlining poly(vinylphosphonic acid) and its derivatives, and in the review of David and Coll. [5], devoted to phosphonate vinyl monomers and polymers. Monomers and PCPAs containing fluoro substituents in the main polymer chain were mentioned in the review of Améduri and coll. [6]. A representative list of vinyl monomers suitable for the preparation of adhesive (co)polymers for dentistry was presented in the work of Moszner, Salz, and Zimmermann [7].
2.2.1. Vinylphosphonic Acid and Related Compounds
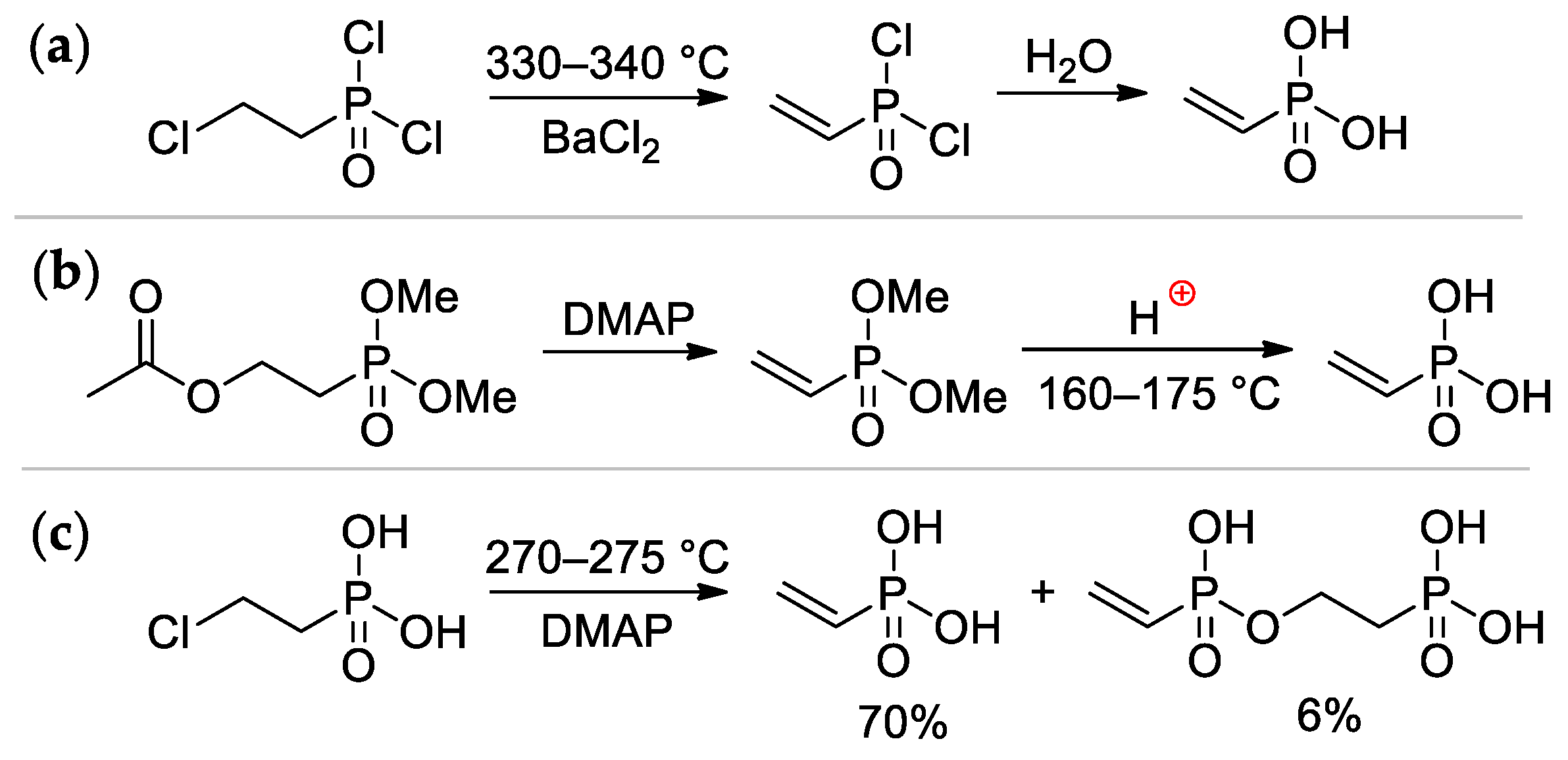
Vinylphosphonic acid (VPA) CH2=CHP(O)(OH)2 represents the simplest monomer for the synthesis of sidechain PCPAs [4]. It was obtained for the first time by Kabachnik and Medved in 1959 via dehydrochlorination of ClCH2CH2P(O)Cl2 (330–340 °C, BaCl2) followed by hydrolysis of CH2=CHP(O)Cl2 intermediate [8] (Scheme 2a); in turn, ClCH2CH2P(O)Cl2 was prepared by the reaction of PCl3 with oxirane, rearrangement of the resulting P(OCH2CH2Cl)3 to ClCH2CH2P(O)(OCH2CH2Cl)2, followed by its reaction with thionyl chloride [9]. Another method was based on the reaction of AcOCH2CH2P(O)(OMe)2 with DMAP, followed by acidic hydrolysis of the CH2=CHP(O)(OMe)2 intermediate [10]. (Scheme 2b). The cost-effective synthetic approach was based on a readily available starting compound ClCH2CH2P(O)(OH)2 (used as a growth regulator for plants), its pyrolysis was accompanied by the formation of the bis(phosphonate) side product [11] (Scheme 2c). The use of microwave irradiation in the pyrolysis of ClCH2CH2P(O)(OH)2 proved ineffective [12]. Vinylphosphonic acid is a colorless, low-melting solid (M.p. 36 °C) [13].
An efficient method of the synthesis of 2-propenylphosphonic acid involves the nucleophilic addition of PCl3 to acetone followed by the reaction with AcOH and subsequent dehydrochlorination [14] (Scheme 3).
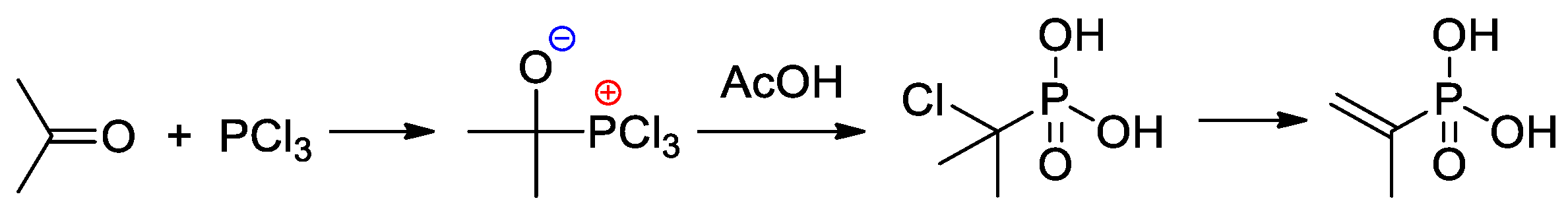
Scheme 3. The synthesis of 2-propenylphosphonic acid [14].
2.2.2. Phosphorylated Acrylates and Related Compounds
The high reactivity of the esters of acrylic and methacrylic acids in polymerization and their greater synthetic availability in comparison with VPA and VPA esters are the reasons for researchers’ continued interest in vinyl monomers of this type. Among others, derivatives of (2-hydroxyethyl)methacrylate (HEMA) deserve special mention due to their synthetic availability.
Different synthetic approaches to phosphate- and phosphonate-functionalized acrylates are presented and discussed below. Evidently, phosphate or phosphonate groups can be introduced as a part of the alkoxy fragment of (meth)acrylate (the easiest and most affordable way, Scheme 4), but functionalization of the methyl group of methacrylate also leads to prospective monomers (Scheme 5).
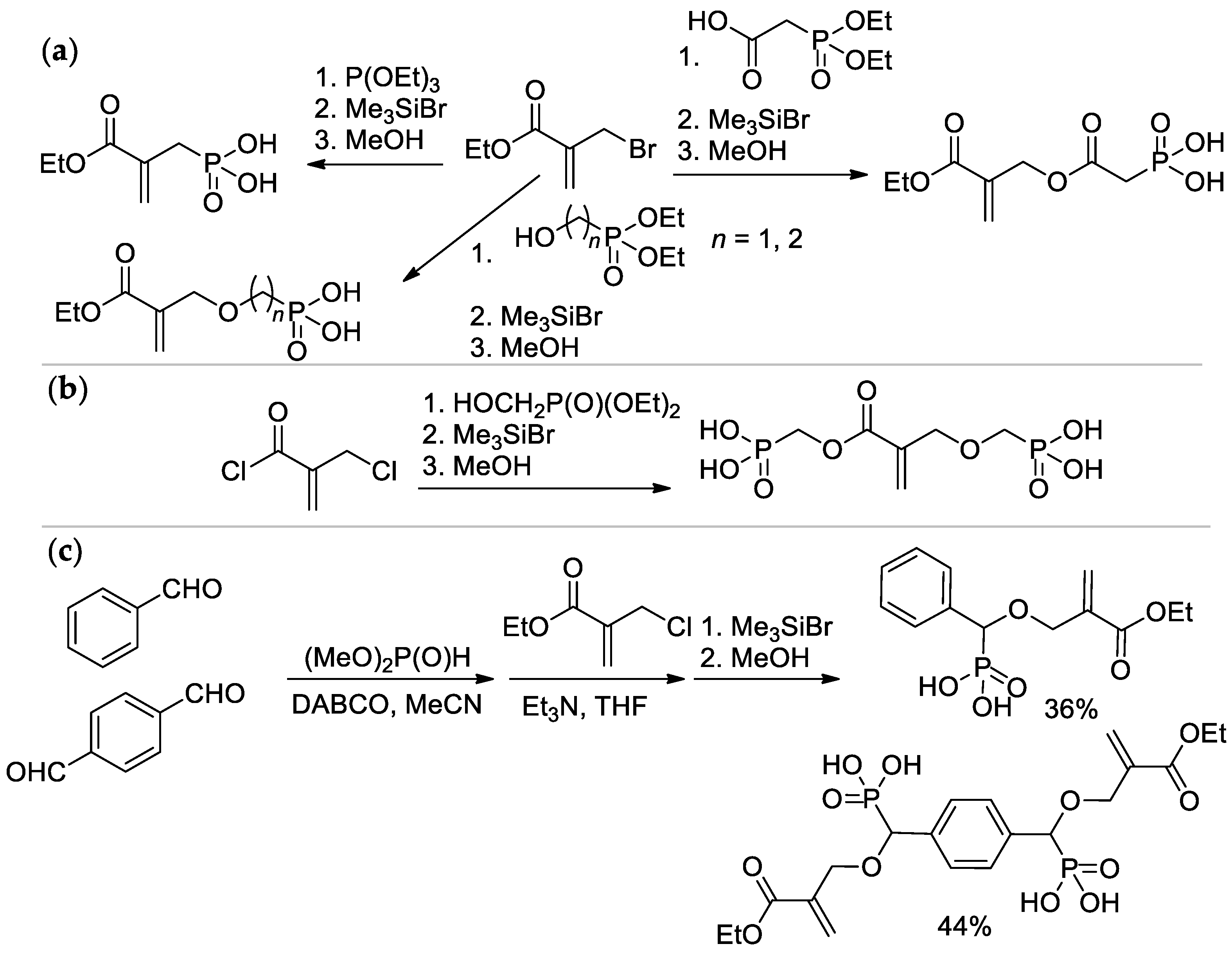
Scheme 5. Synthesis of phosphonated methacrylates via functionalization of the methyl group: (a) based on ethyl 2-(bromomethyl)acrylate; (b) from 2-(chloromethyl)acryloyl chloride; (c) via condensation of aldehydes with dimethyl phosphonate.
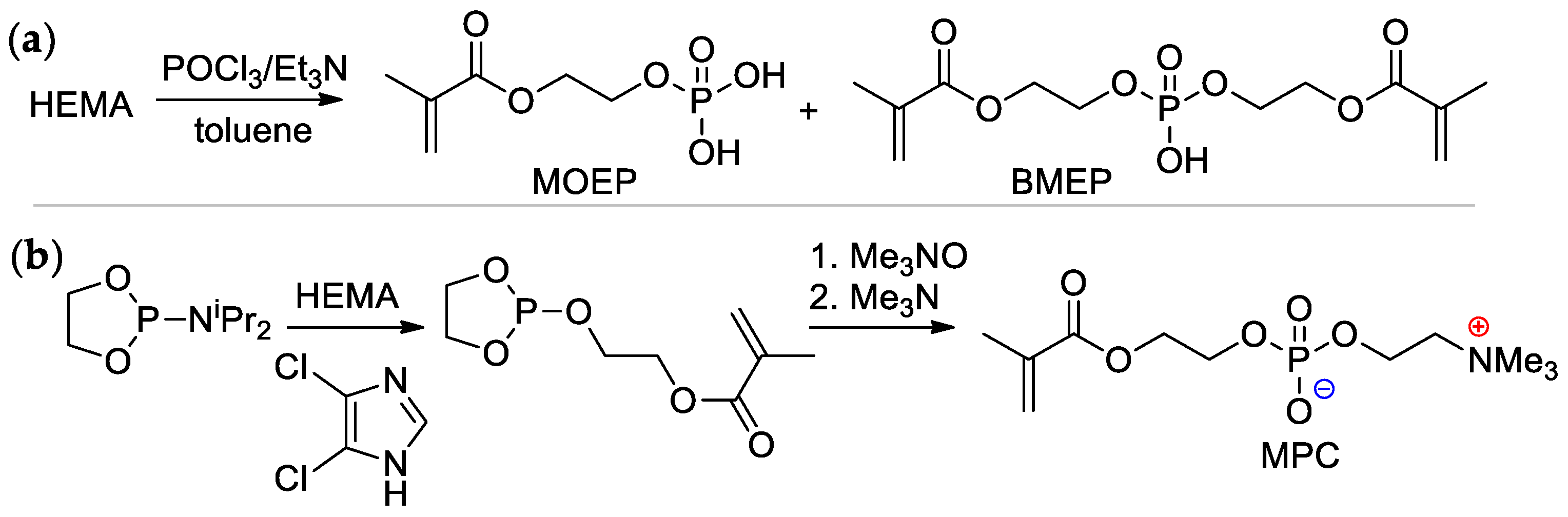
2-(Methacryloyloxy)ethyl phosphate (MOEP) and bis[2-(methacryloyloxy)ethyl]phosphate (BMEP) were synthesized by the reaction of HEMA with POCl3, followed by hydrolysis, the yields of the products depended on the HEMA/POCl3 ratio [15][16] (Scheme 4a). Direct condensation of HEMA with phosphorylcholine is accompanied by polymerization, and the synthesis of methacryloyl phosphorylcholine (MPC) was carried out using the ‘phosphoramidite’ route with an overall yield of 37% [17] (Scheme 4b).
The Michaelis–Arbuzov reaction of α-bromo ethyl methacrylate with P(OEt)3 with subsequent hydrolysis resulted in the formation of (2-(ethoxycarbonyl)allyl)phosphonic acid [15][18], while its acylation by 2-(diethoxyphosphoryl)acetic acid and hydrolysis allowed to obtain (2-((2-(ethoxycarbonyl)allyl)oxy)-2-oxoethyl)phosphonic acid [19]; alkylation and hydrolysis lead to ether-linked acrylic phosphonates [20] (Scheme 5a). Interaction of 2-(chloromethyl)acryloyl chloride with hydroxymethyl diethyl phosphonate afforded bis(phosphinic acid) [18] (Scheme 5b). Synthesis of aromatic acrylic phosphonates was carried out by the Pudovik reaction, starting from benzaldehyde or terephthaldialdehyde [15][20] (Scheme 5c).
As can be seen in Scheme 5b, both alkoxy and methyl groups of methacrylate can be affected by modification. The applicability of this approach was also demonstrated by Avci and coll et al., who used the example of the synthesis of Bisphenol A-based bis(phosphonic acid) (Scheme 6) [21].
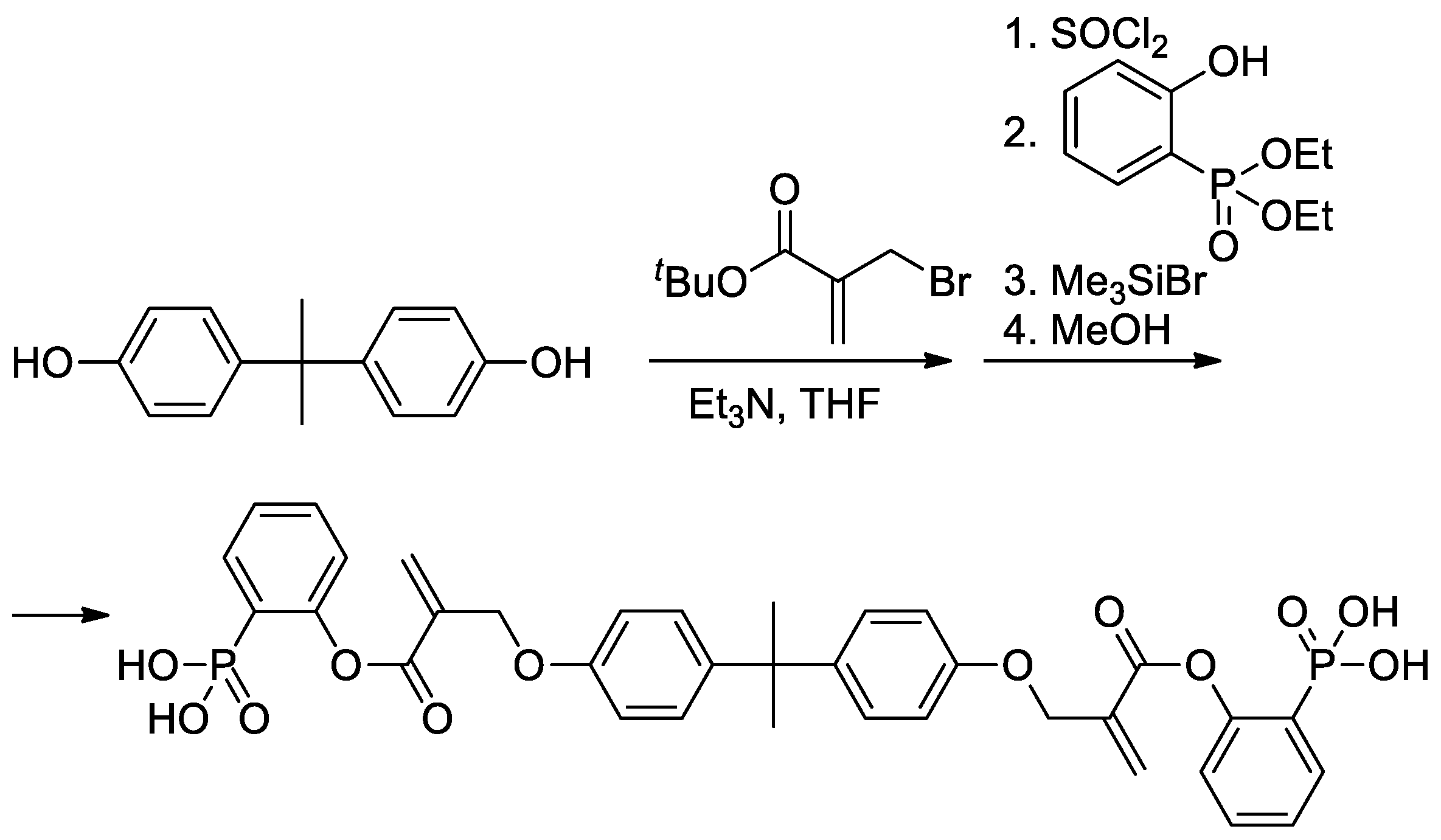
Scheme 6. Synthesis of bis(phosphonic acid) from Bisphenol A [21].
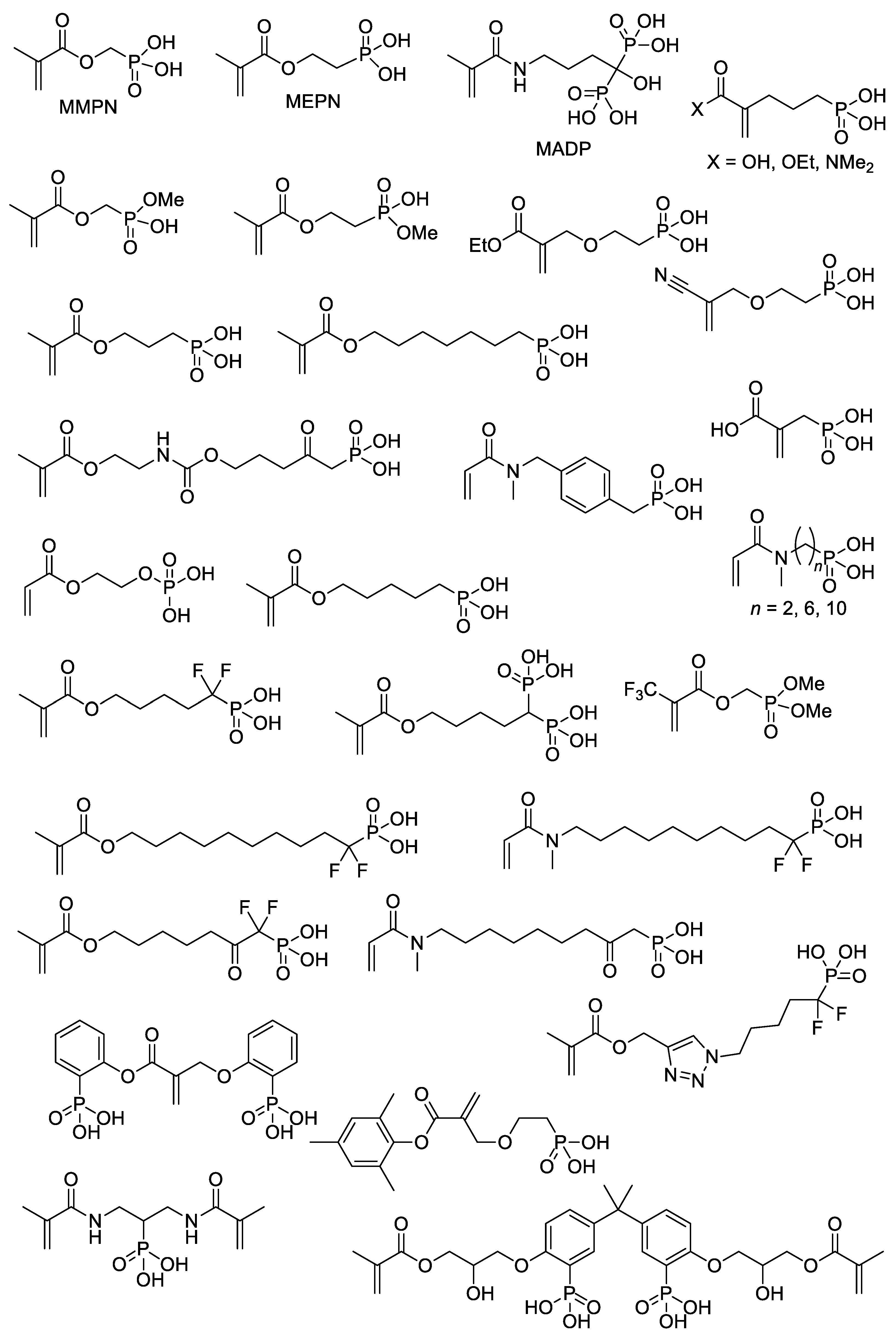
With the use of common synthetic approaches (Scheme 4 and Scheme 5) and other equally simple reactions, dozens of –P(O)(OH)2-functionalized acrylates were synthesized [15][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36] (Scheme 7). An important note is that an incomplete list of acrylates, presented in Scheme 7, can be further expanded by introducing dialkyl phosphonate since deprotection with the formation of PCPAs can be easily carried out after polymerization [37][38]. However, a number of acrylate monomers containing –P(O)(OR)2 groups and polymers obtained on their basis were not studied in the synthesis of PCPAs [39][40].
2.2.3. Other Phosphorus Containing Monomers
Fluorinated polymers represent a separate group of materials due to specific surface characteristics and high thermal and chemical stability. In a recent review by Améduri et al. [6], different synthetic strategies for fluorinated (co)polymers containing phosphorus groups are discussed, with the consideration of –P(O)(OH)2-functionalized monomers and polymers. However, the synthesis of such type compounds was described in a single article by Yamabe et al. who developed a complex and time-consuming method for the synthesis of perfuorovinyloxy-substituted perfuoroalkylphosphonic acid derivatives CF2=CFO(CF2)3P(O)(OMe)2 [41].
Since substituted styrenes are highly active in all types of polymerizations, the idea of using –P(O)(OH)2-functionalized styrenes in polymerization is certainly feasible. Based on 4-(chloromethyl)-styrene, Yoon and Coll. [42] have synthesized styryl phosphonic acids with different lengths of the alkanediyl spacers between the benzene ring and –P(O)(OH)2 group (Scheme 8). To introduce CH2CF2 linkage between styrene and phosphonate fragments, Alter and Hoge used the reaction of 4-(iodomethyl)styrene with LiCF2P(O)(OEt)2 [43].
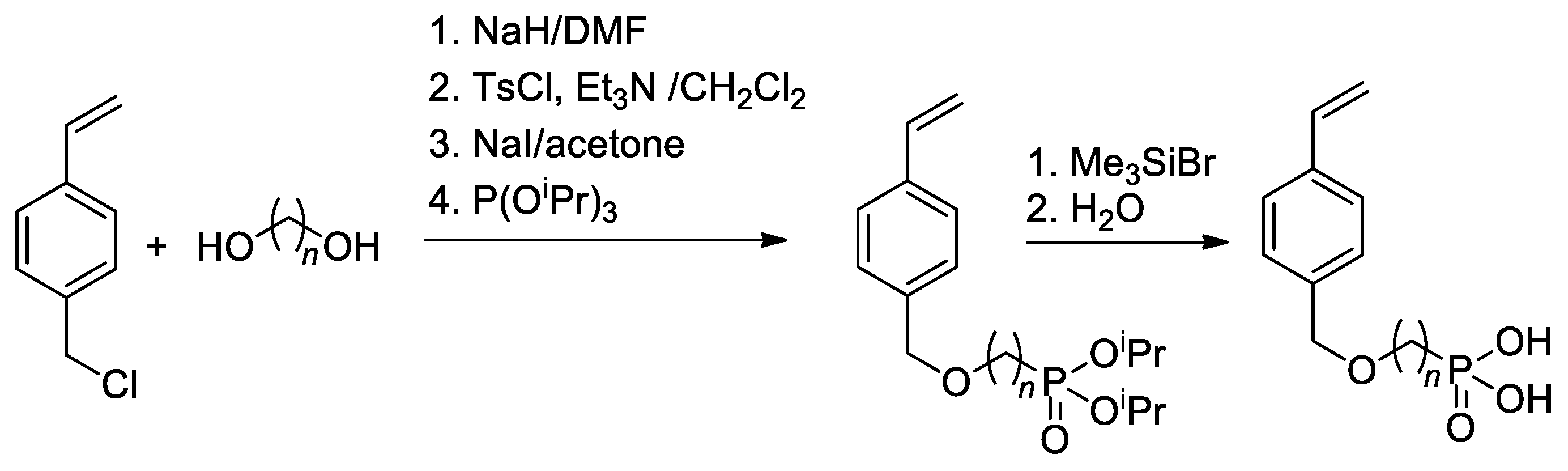
Scheme 8. Synthesis of styrene-derivative monomers containing the phosphonic acid [42].
Later, Park et al. proposed the use of Pd-catalyzed cross-coupling for the direct introduction of one or two –P(O)(OEt)2 fragments into an aromatic ring with a formation of styryl phosphonate and bis(phosphonate) (SP and SbP, respectively) [44] (Scheme 9).
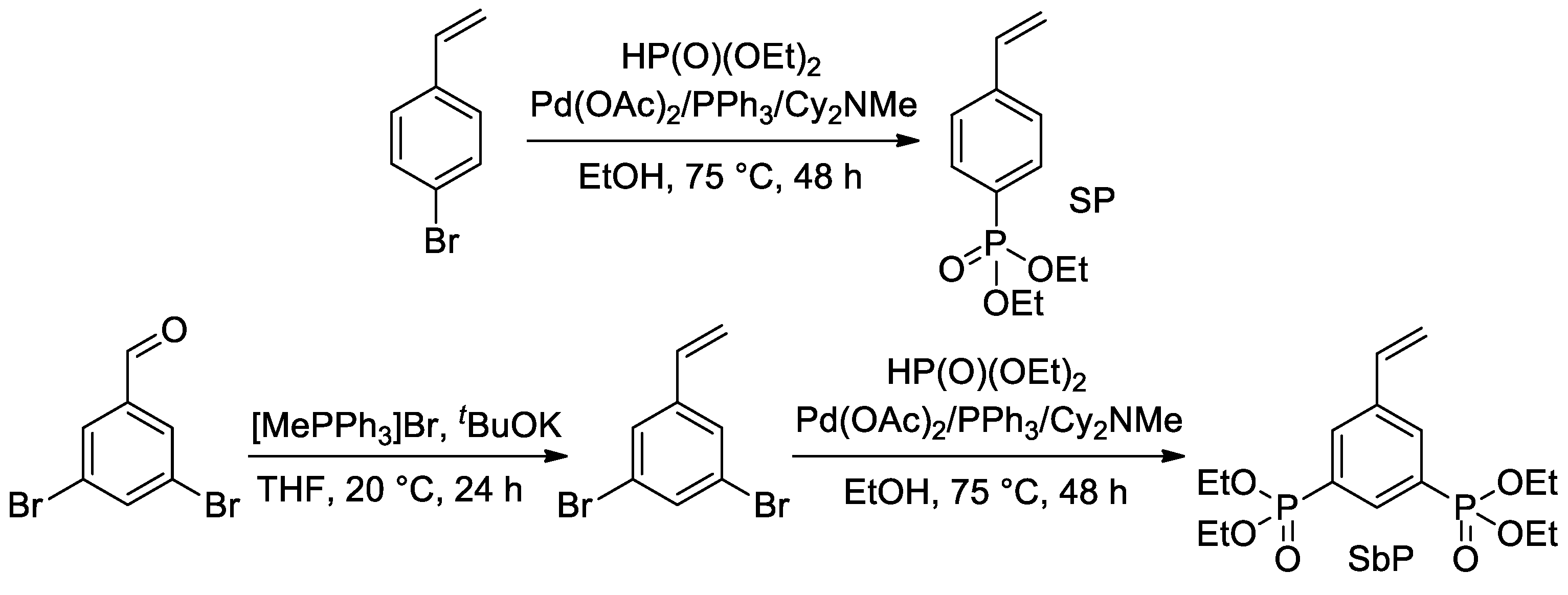
Scheme 9. Synthesis of styrenes with one or two –P(O)(OEt)2 substituents [44].
In the study of Hoge et al. [45], the idea of the use of styrene-derived phosphonates was further developed. Considering the high para-selectivity of nucleophilic substitution in N,N,N′,N′-tetraethyl-1-(perfluorophenyl)phosphinediamine, they synthesized vinyl monomer having 1,4-perfluorophenylene linkage between styryl and –P(O)(OH)2 fragments (Scheme 10). Note that intermediate phosphinic acid is virtually the only phosphorus-containing ‘monomer’ with a proven molecular structure (Figure 1).
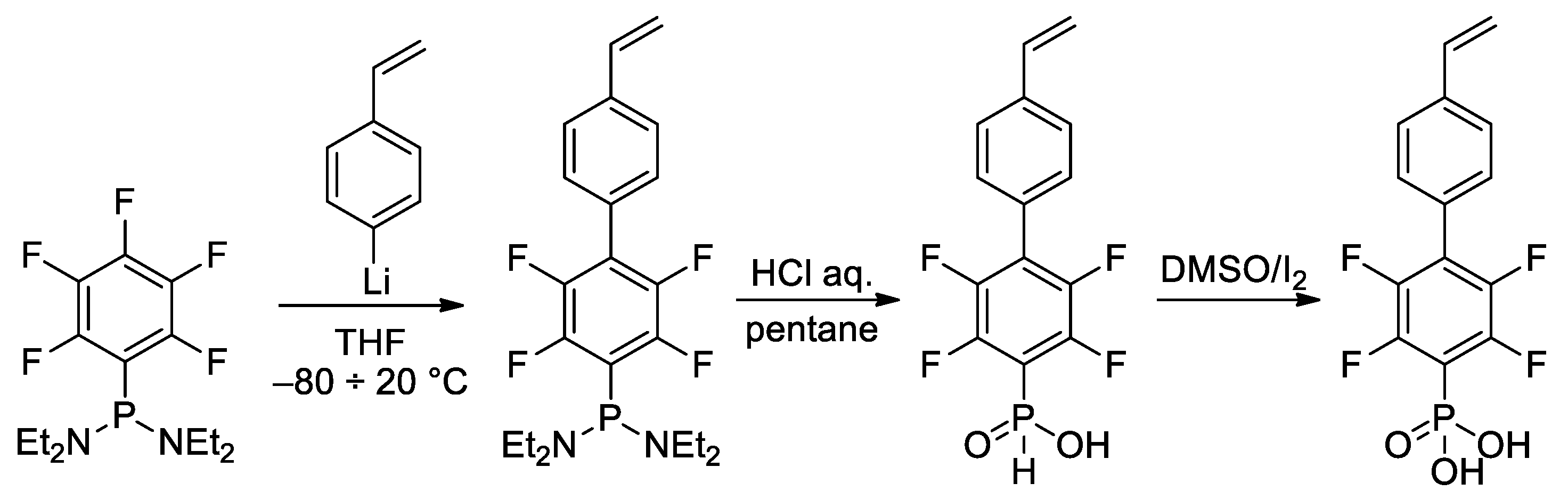
Scheme 10. Synthesis of phosphonic acid with 1,4-perfluorophenylene linkage between styryl and –P(O)(OH)2 fragments [45].
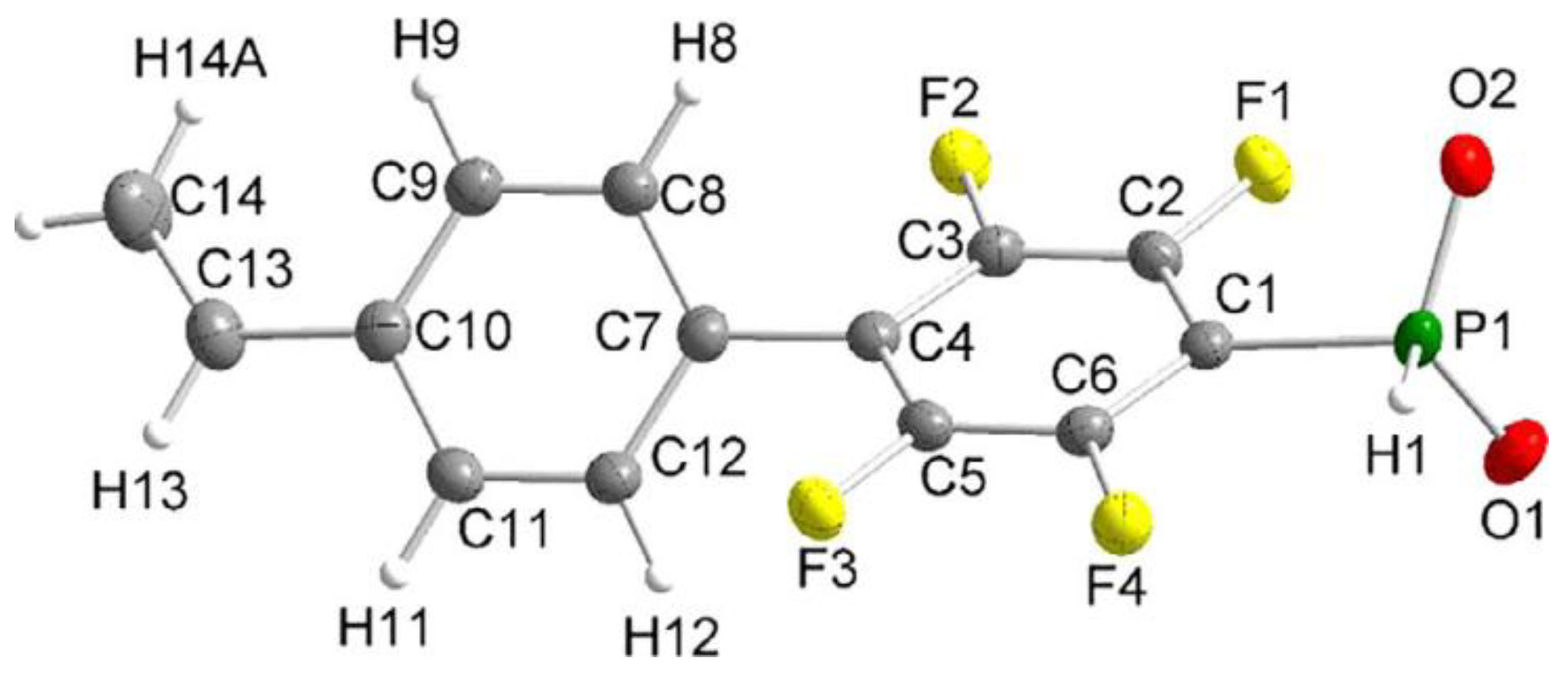
Figure 1. Molecular structure of the anion of the diethyl ammonium salt of phosphinic acid with 1,4-perfluorophenylene linkage between styryl and –PO2H− fragments (thermal ellipsoids are set to 50% probability; cation omitted.). Reprinted with permission from [45]. Copyright (2020) Wiley-VCH GmbH.
Dialkyl(alkenyl)phosphates, readily available by the reaction of enolates with ClP(O)(OR)2 [46][47], can also be considered as monomers for the synthesis of sidechain PCPAs; however, their formation is complicated by the rearrangement to β-ketophosphonates [48][49].
2.3. Homopolymerization and Copolymerization of the Phosphorus Containing Vinyl Monomers
2.3.1. Homopolymerization of VPA, Its Derivatives and Analogs
Since poly(vinylphosphonic acid) (PVPA) continues to attract the researchers’ attention based on new fields of PVPA can be synthesized via radical polymerization of vinylphosphonic acid in the presence of initiators and optionally chain transfer agents (CTAs) with different functionality and reactivity.
Wegner et al. [50] conducted a series of experiments on VPA polymerization in aqueous media using α,α′-azodiisobutyramidine dihydrochloride (AIBA) as an initiator. In contrast to free-radical polymerization of other polar vinyl monomers, polymerization of VPA occurred with low regioselectivity, and a probability of head-to-head and tail-to-tail links over regular head-to-tail links of 23.5% was obtained. When using AIBN as an initiator and ethyl acetate as a solvent, PVPA containing up to 17% head-to-head fragments was obtained [51]. To explain the low regioselectivity of VPA polymerization, Wegner et al. [50] suggested that the process may occur according to two different mechanisms, i.e., a classical head-to-tail radical polymerization and a cyclopolymerization of the VPA anhydride formed in situ (Scheme 11). Further studies on VPA polymerization in Ac2O media, conducted by Voit and Coll. [52], have shown that the intermediate formation of anhydride results in an acceleration of the reaction due to higher reactivity of the VPA anhydride with a formation of cyclic side-products (Scheme 11). The formation of anhydride fragments (up to 19 wt%) was also detected when the polymerization of VPA was conducted in ethyl acetate with the use of a benzoyl peroxide initiator [53]. AIBA-initiated homopolymerization of VPA was used in the preparation of PVPA samples suitable for composition with different inorganic compounds [54][55][56].
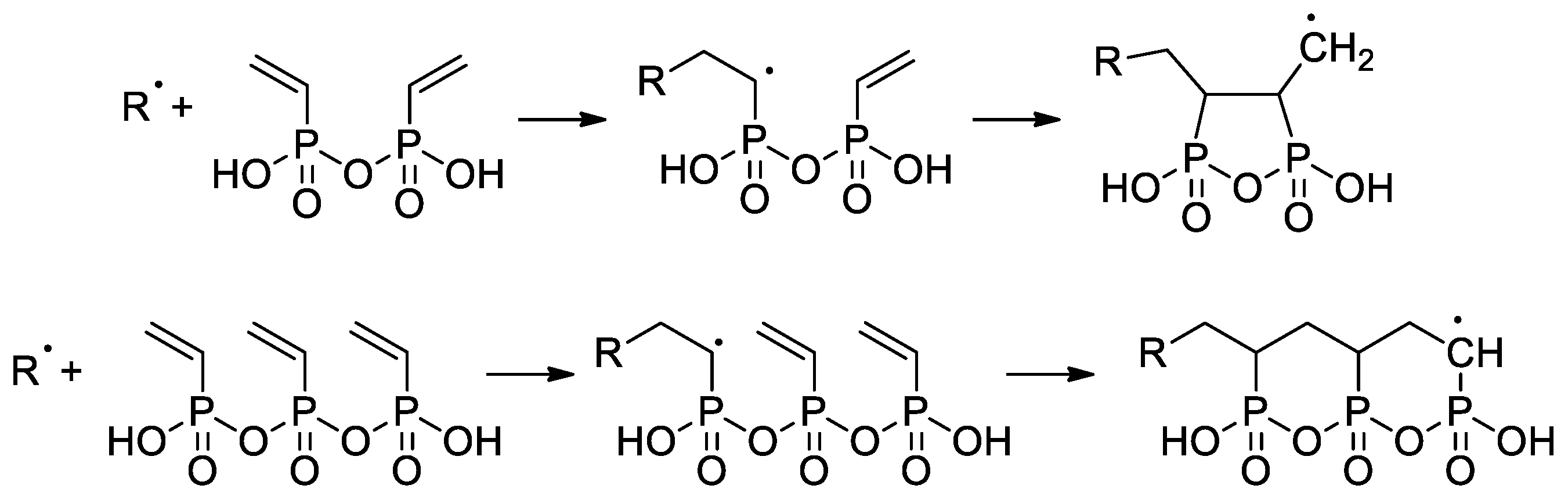
The use CTAs in the radical polymerization of VPA was reported for the first time by David and Coll. [57]. Only CBrCl3 successfully served as CTA in the synthesis of telechelic VPA oligomers with yields of ~70% and DPn = 6–60. High-MW PVPA (Mn 42.2 kDa) was obtained in aqueous media at 80 °C when using a 620:1 VPA/AIBA ratio [58].
Prospective results were received when using n-C6F13I as a CTA that exhibited CF2I functionalization of the oligomer obtained. MALDI-TOF MS and NMR analysis indicated that conventional radical initiation and termination do not occur and that the oligomers were obtained from transfer reactions [59].
The applicability of reversible addition-fragmentation chain transfer/macromolecular design via the interchange of xanthate (RAFT/MADIX) approach to radical polymerization of VPA was studied by Destarac and Coll. [60]. They demonstrated that at 65 °C in water carboxy functionalized O-ethyl xanthate reacts rapidly and quantitatively with VPA oligoradicals to yield low-MW O-ethyl xanthate-capped PVPAs (Scheme 12), and later, reversible transfer of the xanthate terminal group leads to an increase of Mn during polymerization. AIBA was used as a water-soluble initiator of radical polymerization. A follow-up study by the same group has shown that in the presence of 0.25-0.75, molar equivalents of NaOH, the rate and the final conversion of both conventional and RAFT polymerizations were increased, and the fastest rates of polymerization were obtained in the presence of 0.5 equivalents of NaOH [61].
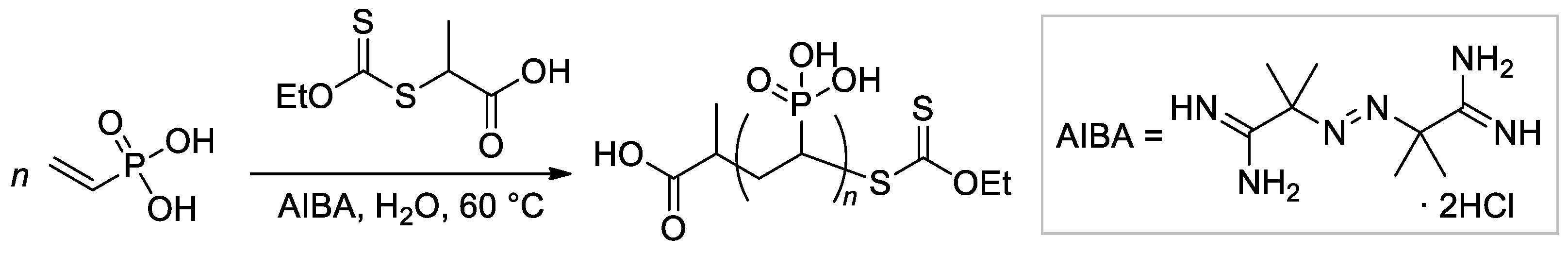
Scheme 12. Aqueous RAFT/MADIX polymerisation of VPA mediated by a carboxy-functional xanthate [60].
The other possibility to obtain PVPA consists in the polymerization of vinylphosphonic acid derivatives CH2=CHP(O)Cl2 or CH2=CHP(O)(OR)2 followed by hydrolysis. As was demonstrated by Ellis and Wilson, PVPA can also be obtained by free-radical polymerization of CH2=CHP(O)Cl2 with the use of an AIBN initiator, followed by hydrolysis [62]. However, to provide acceptable mechanical characteristics of PVPA-based materials, the relatively low-MW polymer thus obtained was forcedly subjected to the treatment with cross-linking reagents [63].
Jin and Gonsalves reported a relatively low Mn value of 8 kDa for homopolymer formed during AIBN-initiated bulk polymerization of CH2=CHP(O)(OMe)2 [64], the microstructure of the polymer was not determined. During the study of AIBA-initiated polymerization of CH2=CHP(O)(OMe)2, Wegner et al. [50] showed higher regioselectivity of this reaction in comparison with VPA homopolymerization. For the transformation of the polymer obtained into PVPA, it reacted with an excess of aq. HBr at 110 °C for 8 h. Note that radical polymerization of CH2=CHP(O)(OiPr)2 can be complicated by intramolecular hydrogen transfer from the backbone to the side chain [65] (Scheme 13).
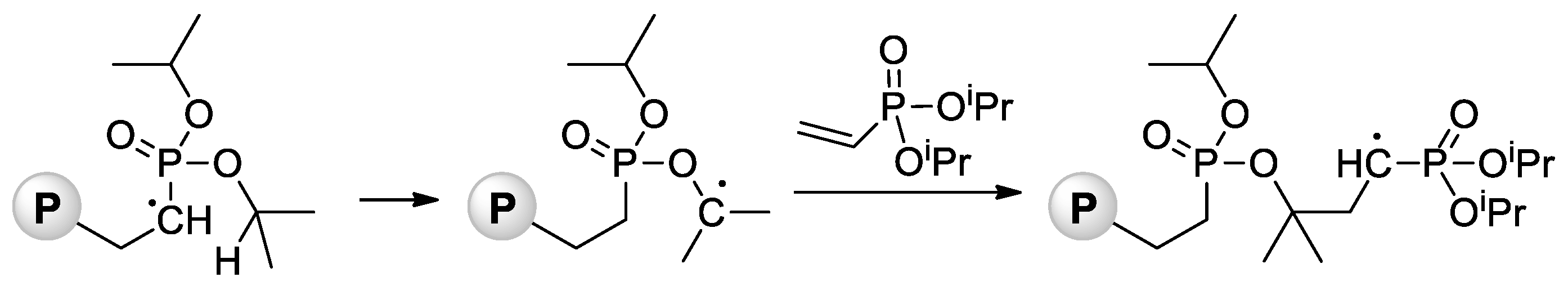
Scheme 13. Intramolecular hydrogen transfer from the backbone to the side chain with formation of P–O–C bonds during radical polymerization of diisopropyl vinyl phosphonate (P = polymer) [65].
The study of UV-induced copolymerization of VPA with CH2=CHP(O)(OMe)2 [66] deserves to be mentioned: in the presence of 3 wt% Darocur 4265 photoinitiator (that represents a mixture of (diphenylphosphoryl)(mesityl)methanone and 2-hydroxy-2-methyl-1-phenylpropan-1-one), VPA or VPA/phosphonate mixtures were laid on PTFE plates and exposed to UV irradiation. PVPA with Mn = 27.75 kDa (ÐM = 1.23) was obtained; for copolymers, Mn values increased from 8.78 to 16.12 kDa with an increase in the VPA/CH2=CHP(O)(OMe)2 ratio from 1:1 to 4:1. ATRP was also used in the synthesis of core-shell materials with the use of oligo-(CMe2Br) substituted polyaromatics and CH2=CHP(O)(OEt)2 [67].
The relatively low reactivity of CH2=CHP(O)(OR)2 in free-radical polymerization has heavily conditioned the search for alternative methods of polymerization of dialkyl vinyl phosphonates. Meyer and Coll. proposed the use of sBuLi and Ph2C=CH2 as an initiator of the anionic polymerization of CH2=CHP(O)(OR)2 (R = Me, iPr) in THF [68]. High yields of the polymers were obtained when using diisopropyl vinyl phosphonate, while CH2=CHP(O)(OMe)2 gave only low yields due to the precipitation of the polymer already in low conversions. Anionic polymerization proceeded regioselectively, but with low stereoselectivity. Given that mild and quantitative hydrolysis of dialkyl phosphonates can be performed via the intermediate formation of silyl esters [69], polyesters were refluxed with Me3SiBr in CH2Cl2 to yield PVPA samples of different Mw (4.2–814 kDa) and ĐM of 2.1–3.9 (Scheme 14).
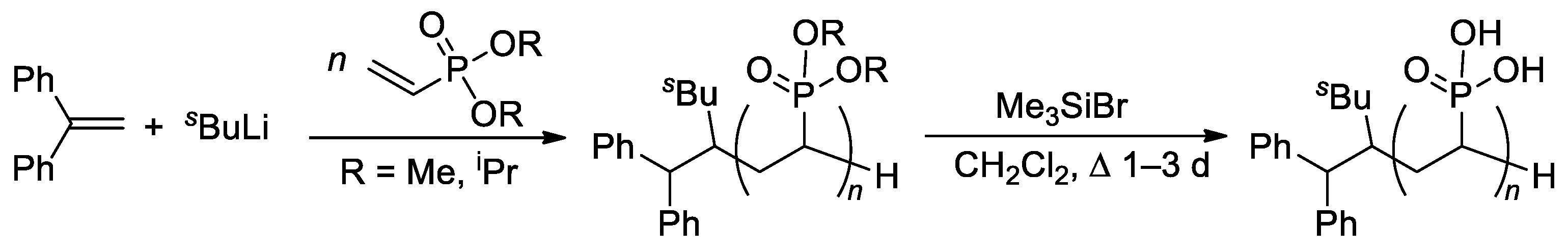
Scheme 14. Synthesis of PVPA by anionic polymerization of dialkyl vinyl phosphonate, followed by the cleavage using Me3SiBr [68].
A similar approach to PVPA via anionic polymerization of CH2=CHP(O)(OMe)2 with subsequent hydrolysis was implemented by Takeichi et al. [70] with the use of tBuLi or tBuLi/nBu3Al (1:5 mol/mol) as an initiator at the first stage (polymerization was conducted in toluene media), and conc. HCl with 12 h of reflux at the second stage. When polymerization was initiated by tBuLi/nBu3Al instead of tBuLi alone, a threefold increase in monomer conversion was detected and, more significantly, ÐM decreased by half, from 2.65 to 1.30, and isotacticity increased. The water solutions of the PVPAs were cast on a glass plate and allowed to stand at room temperature for seven days. After drying, the PVPA (Mn = 5.0 kDa, m = 52%), prepared through the radical polymerization, became viscous liquids, whereas PVPA (Mn = 5.5 kDa, m = 67%), prepared through the anionic process, formed self-standing transparent film. In this way, the quality of the PVPA obtained at –78 °C with the use of tBuLi/nBu3Al initiator is clearly—in the proper sense of the word—illustrated by Figure 2.

Figure 2. Photograph of a PVPA film derived from PDMVP prepared with tBuLi/nBu3Al at −78 °C. Reprinted with permission from [70]. Copyright (2010) Wiley-VCH GmbH.
Both free-radical and anionic polymerization of CH2=CHP(O)(OR)2 are usually not able to provide high monomer conversions and desirable molecular weight characteristics and microstructure of the polymer. In polyolefin chemistry, a similar problem was effectively solved by the use of coordination catalysis. However, conventional Ziegler-Natta and single-site catalysts, suitable for α-olefin polymerization, cannot be applied in the coordination polymerization of phosphonates due to the high electron donation ability of the oxygen atom of →P=O fragment. To date, only rare earth metal complexes were used successfully to polymerize dialkyl vinyl phosphonates.
In 2010 [71], Rabe and Coll. reported that rare earth metal amides of the formula [N(SiHMe2)2]3(THF)2 (Ln = La, Nd, Sm) catalyze polymerization of CH2=CHP(O)(OEt)2 with a formation of high-MW isotactic polymers (Mn = 65–84 kDa, ÐM = 3.1–3.6, mm = 66–79%). A corresponding Y complex was found to be inactive in polymerization, forming a stable adduct [N(SiHMe2)2]3[CH2=CHP(O)(OEt)2]2. In the same year, Rieger and Coll. described the first example of metallocene-catalyzed polymerization of CH2=CHP(O)(OEt)2 [72]. The complexes (η5-C5H5)2YbX (Cp2YbX, X = Cl, Me) have demonstrated high activities in toluene reaction media, polymerization had the ‘living’ character, and Mw up to 1280 kDa were achieved. Already in this first work, Rieger et al. proposed a group-transfer mechanism of polymerization (Scheme 15) that was confirmed and discussed in subsequent research articles [73][74][75][76][77][78][79] and reviews [80][81] of this scientific group.
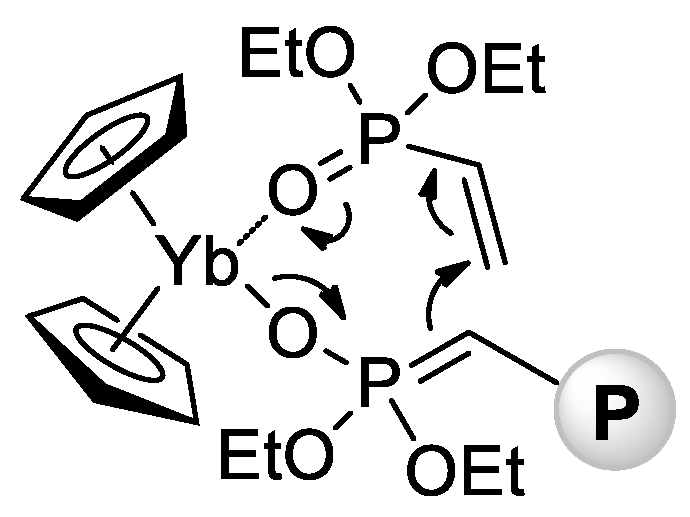
Scheme 15. Postulated intermediate in the group-transfer mechanism of CH2=CHP(O)(OEt)2 polymerization, catalyzed by Cp2YbX. [72].
When using Cp3Ln complexes (Ln = Lu, Yb, Tm, Er, Ho, Dy) as coordination catalysts of CH2=CHP(O)(OEt)2 polymerization, C5H5 fragment acted as an initiator of the chain growth (Scheme 16) [73]. Polymerization had the ‘living’ character, polymers with given Mn (60–710 kDa depending on monomer-to-catalyst ratio) and ÐM = 1.05–1.36 were obtained. Corresponding PVPAs were then prepared for the reaction with Me3SiBr, followed by mild hydrolysis (HCl/MeOH). Dimethyl and diisopropyl vinyl phosphonates were also polymerized. DCS studies have shown that CH2=CHP(O)(OR)2-based polymers decompose at 280–340 °C (R = Et) and 245–270 °C (R = iPr) with olefin elimination and formation of PVPA. When using Cp3Yb catalyst, highly statistic copolymers of CH2=CHP(O)(OR)2 (R = Me, Et, nPr) were obtained [74].
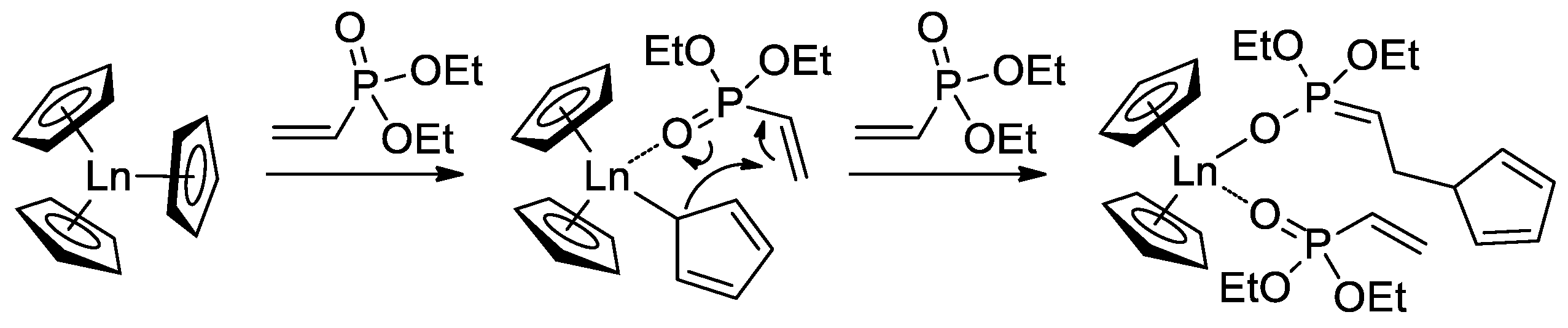
Scheme 16. Initiation of the Cp3Ln-induced polymerization of CH2=CHP(O)(OEt)2 [73].
Since Cp2YbX-catalyzed polymerization of acrylates and CH2=CHP(O)(OEt)2 proceed according to similar group-transfer mechanisms, a surface-initiated group transfer polymerization (SI-GTP), which allows the covalent modification of solids with dense poly(vinylphosphonate) brushes, was carried out [82]. Silicon surfaces were modified with methacrylate functionalities, either via a self-assembled monolayer of 3–(trimethoxysilyl)propyl methacrylate or via a poly(ethylene glycol dimethacrylate) film (prepared by self-initiated photografting and photopolymerization), the surfaces were treated with Cp2YbMe, and then by vinyl phosphonate monomer. After Me3SiBr and Me3SiBr treatment, hydrophilic PVPA surfaces formed. A similar approach was realized using cross-linked polystyrene microspheres [83] (Figure 3).
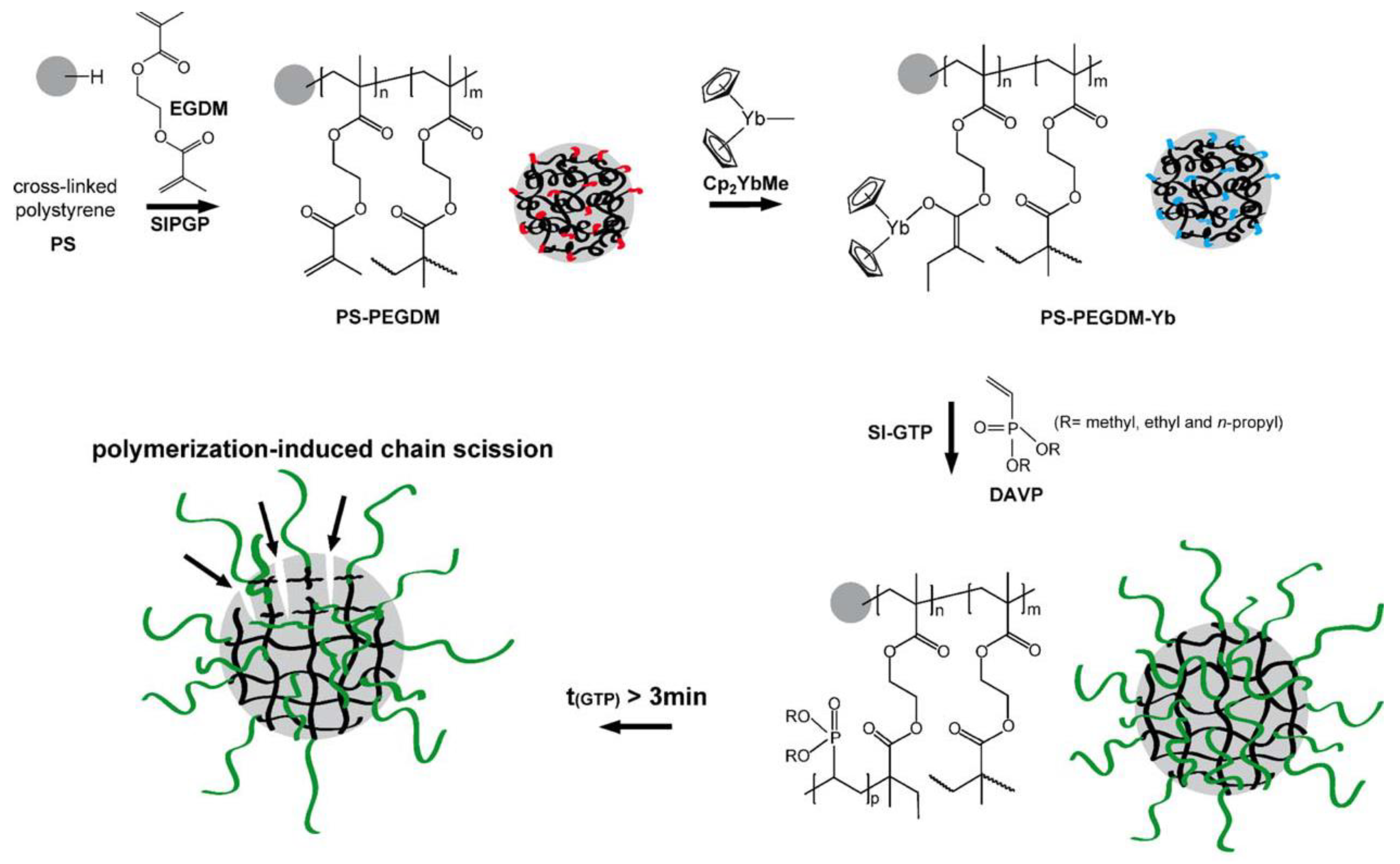
Figure 3. Schematic illustration of the covalent grafting of a PEGDM on a cross-linked polystyrene microsphere and subsequent immobilization of the Cp2YbMe. In the next step, SI-GTP of dialkyl vinyl phosphonate leads to the formation of poly(dialkyl vinyl phosphonate)-functionalized microspheres. Reprinted with permission from [83]. Copyright (2014) Wiley-VCH GmbH.
As a result of the mechanistic studies of polymerization of CH2=CHP(O)(OR)2 [75] it was demonstrated that initiation by Cp2LnX follows a complex reaction pathway depending on the nature of X. For X = Me, CH2SiMe3 initiation occurs via the abstraction of the acidic α-CH of the vinylphosphonate, and for X = Cp, SR initiation occurs via the nucleophilic transfer of X to a coordinated monomer. For X = Cl, OR a monomer-induced ligand-exchange reaction with a formation of active Cp3Ln catalyst was observed. The further elemental steps of the reaction are presented in Figure 4 [75].
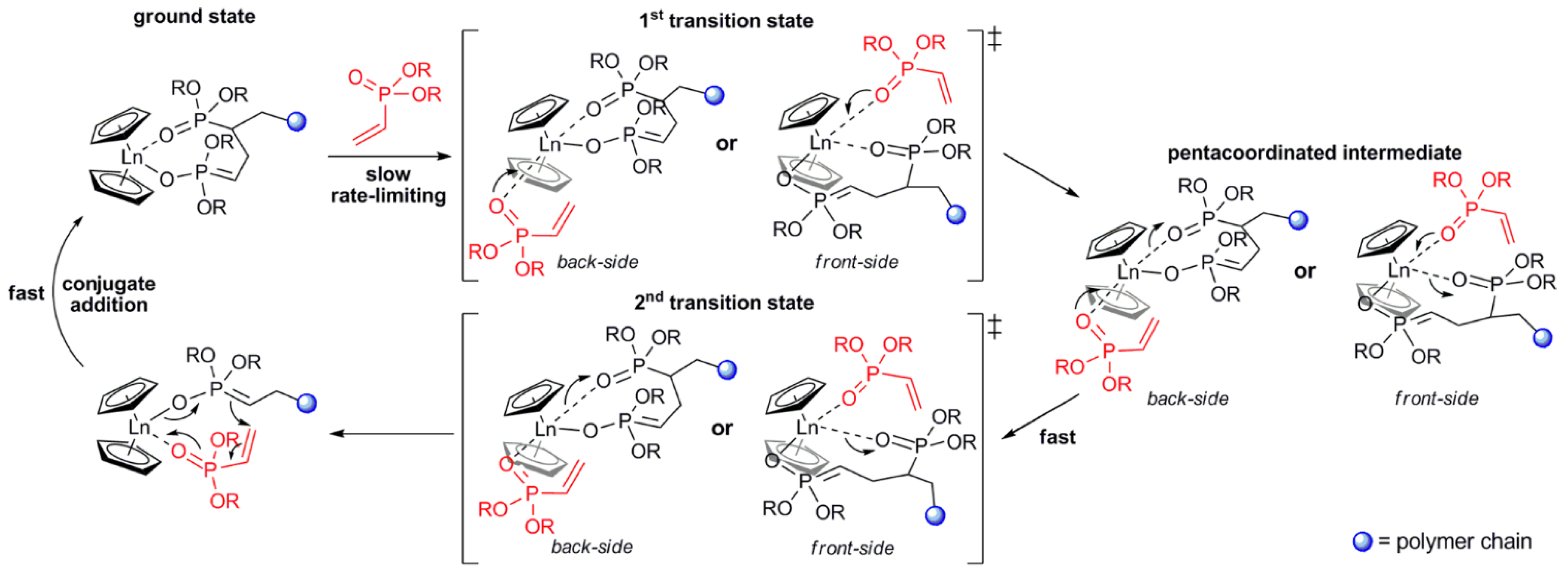
Figure 4. Elemental steps of rare earth-mediated group transfer polymerization of vinyl phosphonates. The rate-limiting step is an SN2-type associative displacement of the polymer phosphonate ester by a vinylphosphonate monomer, presumably via a pentacoordinated intermediate. Reprinted with permission from [75]. Copyright (2013) American Chemical Society.
Since the initiation stage of Cp2LnX-mediated polymerization of CH2=CHP(O)(OR)2, this stage is significantly slower than propagation stages (leading to the broadening of MWD and partial loss of the polymer chain control), subsequent studies were focused on the development of highly efficient initiators. In that capacity, Cp2Ln derivatives of 2,4,6-trimethylpyridine were proposed [76]. The first stage of the process is presented in Scheme 17. Polymerization experiments have demonstrated the absence of the induction period when using this new initiator.
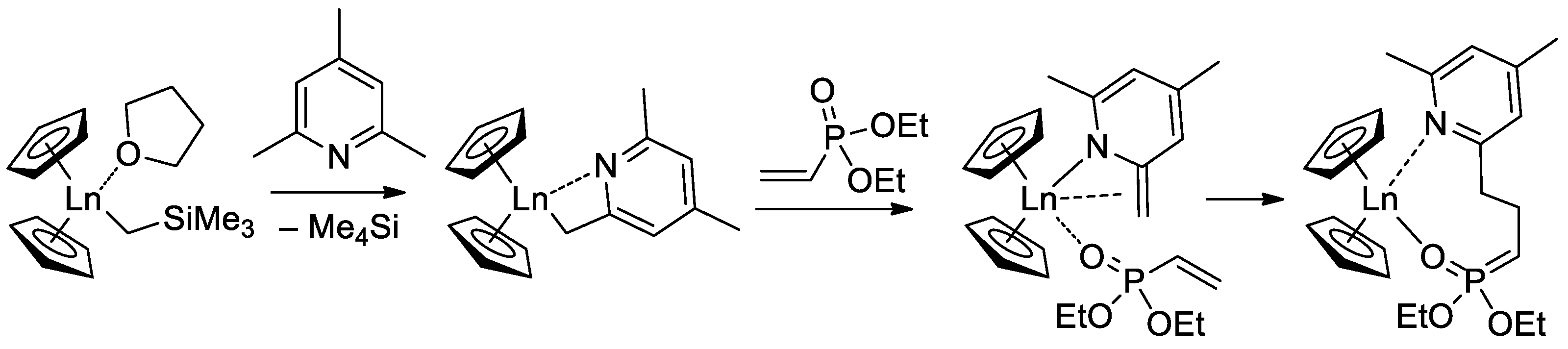
Scheme 17. Synthesis of Cp2Ln(CH2(C5H2Me2N)) via C−H bond activation by σ-bond metathesis and initiation step of the CH2=CHP(O)(OEt)2 polymerization [76].
The concept of the efficient initiator of rare earth metal-mediated group transfer polymerization was beautifully realized by the example of the core-first polymer synthesis [78]. Based on 1,3- (or 1,4-bis-) and 1,3,5-tris(2,6-dimethylpyridin-4-yl)benzene, Cp2Y complexes (Scheme 18) were obtained and used as initiators of the group transfer polymerization of CH2=CHP(O)(OEt)2. When using dinuclear complexes, bimodal polymer distribution differing in molecular weight by a factor of two was detected. In the case of the trinuclear complex, trimodal molecular weight distribution was observed.
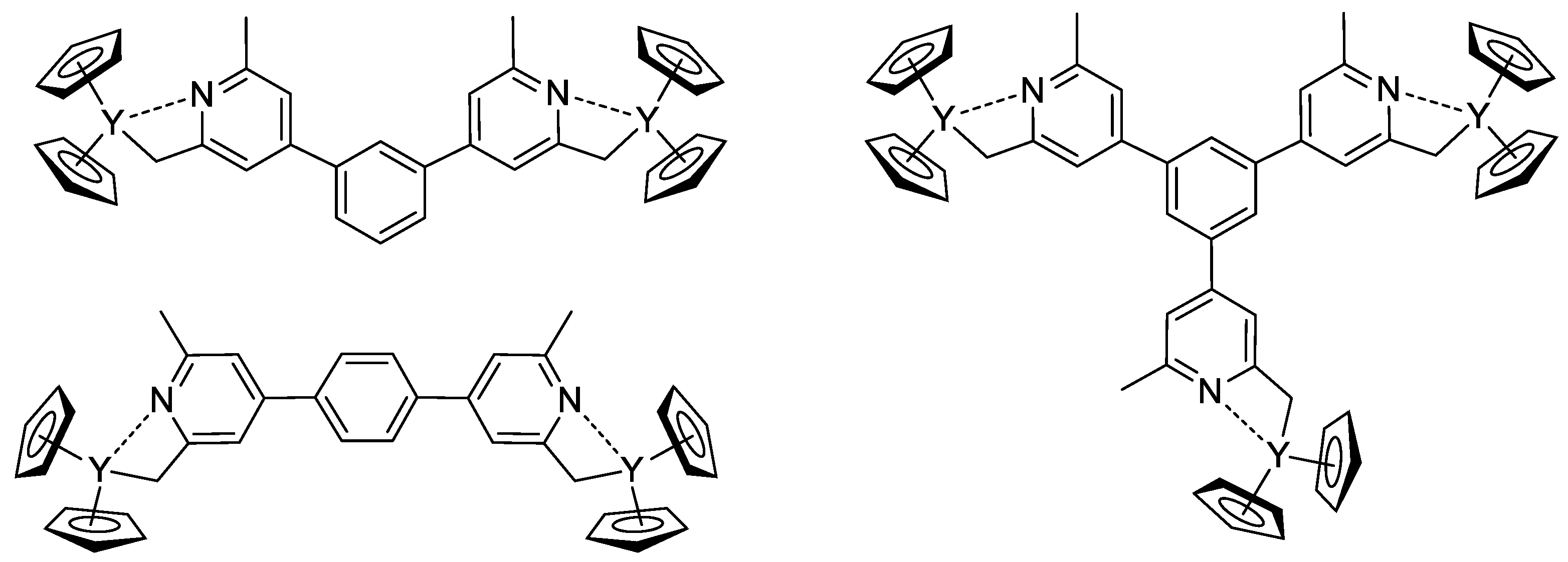
Scheme 18. Efficient initiators for two- and three-pronged polymerization of CH2=CHP(O)(OEt)2 [78].
Rieger and Coll. also drew attention to another important aspect of lanthanidocene-initiated polymerization of CH2=CHP(O)(OR)2, namely, steric factors (Figure 5) [77]. As a result of the metal−ligand interactions, the studied catalysts varied in their properties, ranging from inert (Cp3Ln, Ln = Tb, Sm) to highly active ((C5H4Me)3Y. (C5Me4H)3Sm was the first example of the active ‘early’ lanthanidocenes, and in general, the activities accelerated with decreasing cation size. Furthermore, the polymerization behaviors of (C5Me4H)3Ln complexes differed from unsubstituted or monosubstituted metallocenes. The pentacoordinate intermediates of these complexes exhibited lengthened metal-monomer bond lengths, resulting in a higher enthalpy ΔH≠ term, and are partly compensated by lower ΔS≠ contributions to the activation barrier.
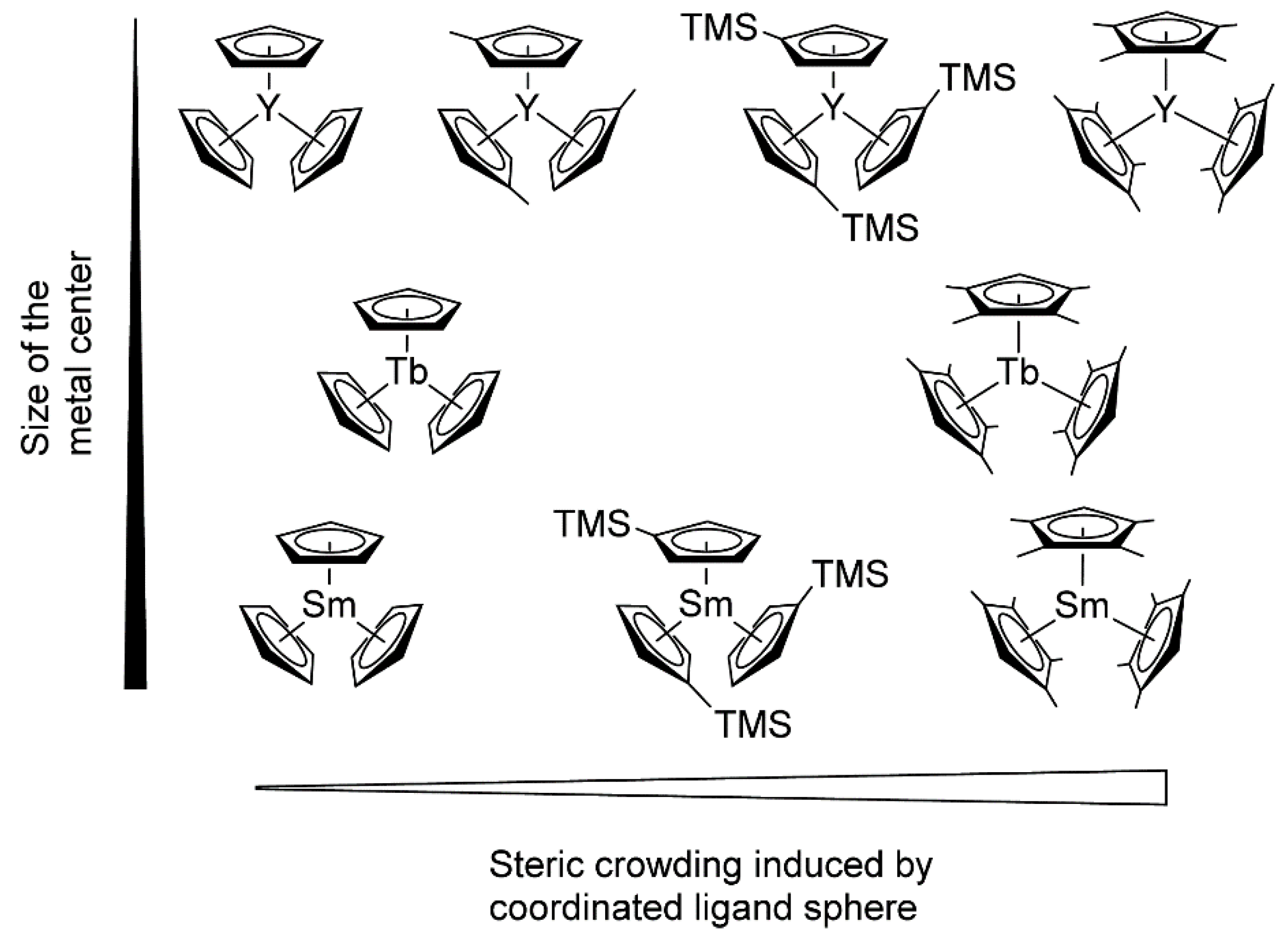
Figure 5. Molecular structures of monosubstituted and tetramethyl-substituted Cp3Ln catalysts studied in polymerization of CH2=CHP(O)(OEt)2. Reprinted with permission from [77]. Copyright (2016) American Chemical Society.
Rare-earth metal-based catalysts of the polymerization of vinyl phosphonates are not limited by sandwich lanthanide complexes. The high catalytic activity of the yttrium bis(phenolate) in the polymerization of CH2=CHP(O)(OR)2 (R = Et, iPr) was demonstrated [84], 2-vinylpyridine efficiently activated the pre-catalyst at the initiation stage (Scheme 19).
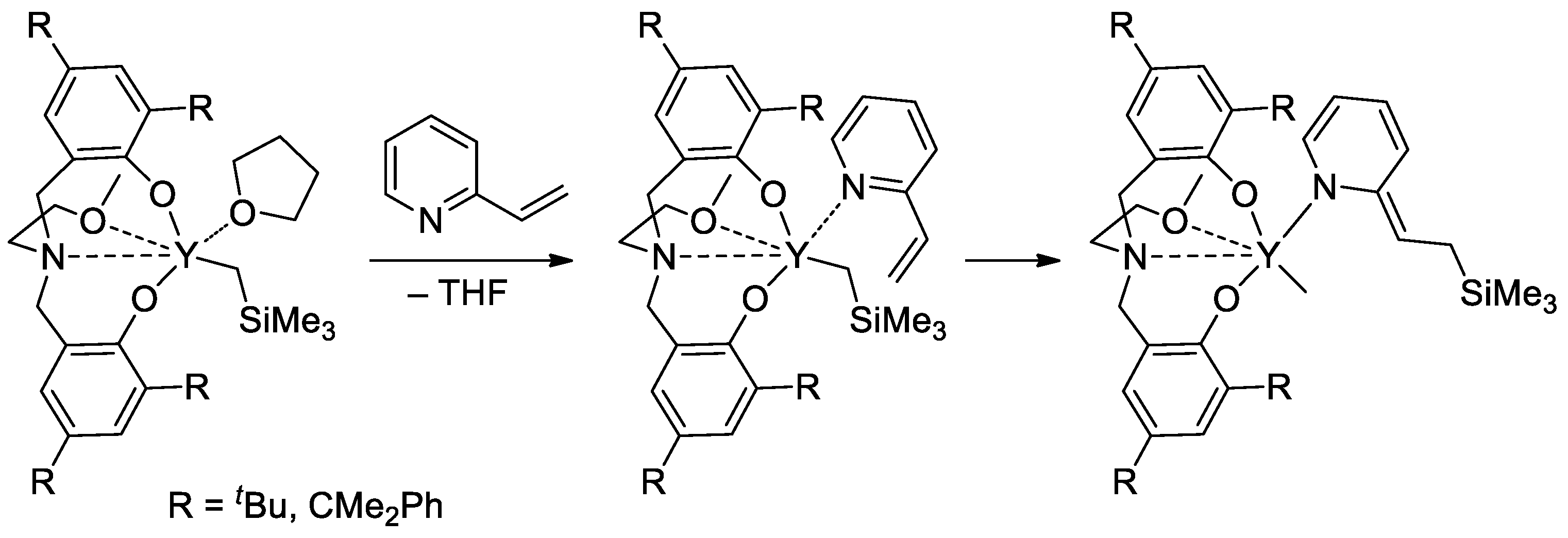
Scheme 19. Six-membered initiation mechanism for 2-vinylpyridine and Y chelate complexes in polymerization of CH2=CHP(O)(OR)2 (R = Et, iPr) [84].
In their recent publication, Rieger and Coll. described the synthesis and catalytic behavior of half-sandwich ‘constrained geometry’ complexes of Y and Al in the polymerization of CH2=CHP(O)(OEt)2 [79]. The complexes of Y (Scheme 20) have demonstrated high activity even at −78 °C, under these conditions highly isotactic polymer (mmm > 98%) was obtained. The proposed stereocontrol mechanism is presented in Figure 6.
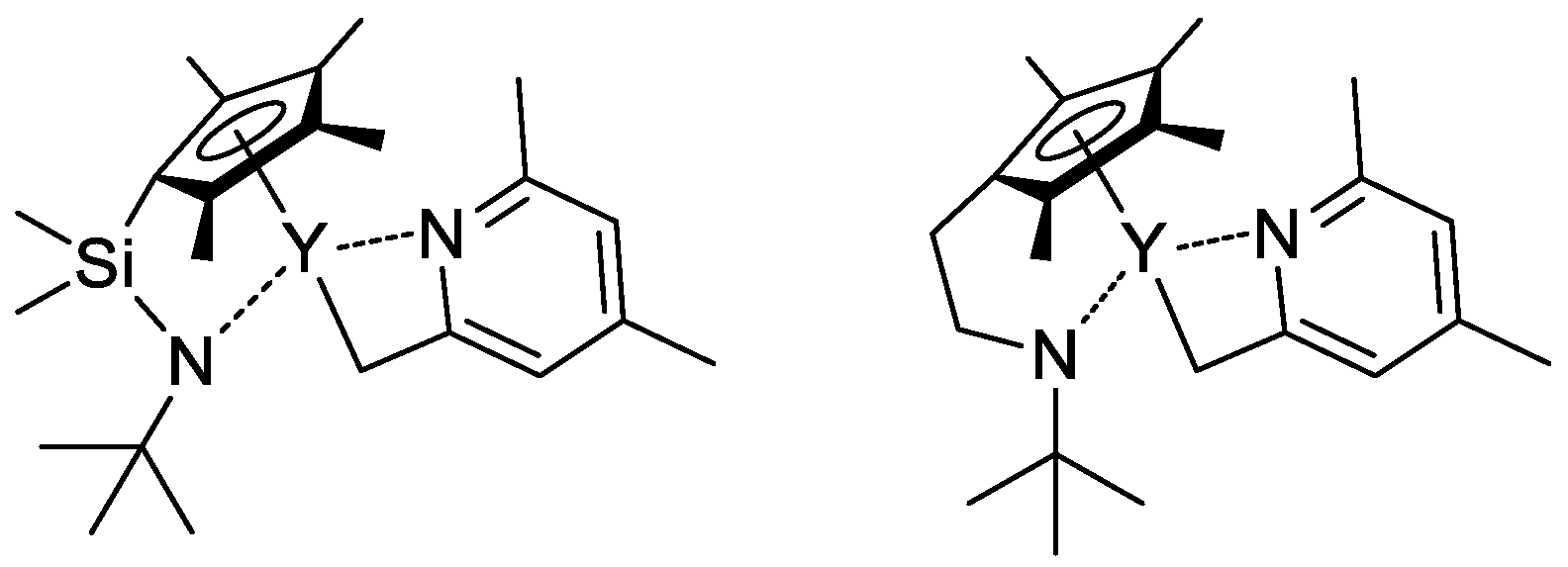
Scheme 20. Efficient constrained-geometry catalysts for ‘living’ polymerization of vinyl phosphonates [79].
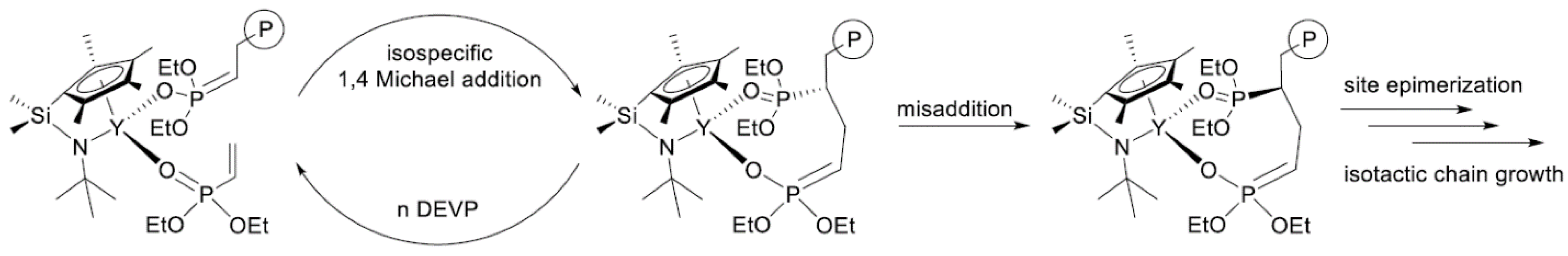
Figure 6. Proposed stereocontrol mechanism and formation of stereoerrors for the isospecific polymerization of CH2=CHP(O)(OEt)2. Reprinted with permission from [79]. Copyright (2019) American Chemical Society.
Note that in 2013 [85], Ni and Coll. have demonstrated high catalytic activity of Ln(BH4)3(THF)3 (Ln = Y, La, Nd, Sm, Gd, Dy, Lu) in group transfer polymerization of CH2=CHP(O)(OEt)2. ‘Living’ character of the reaction was confirmed by the termination of the polymerization with the use of PhCH2Cl or EtI, however, obtained polymers were characterized by broadened MWD (ÐM = 1.63–1.81).
In conclusion, it is important to notice that the treatment with Me3SiBr, followed by mild hydrolysis, or multi-hour reflux in HCl is not the only method for transforming poly(phosphonates) to PVPA. High efficiency of the use of CF3SO3H or highly acidic cationites in ‘wet’ (hydrolysis) and ‘dry’ (olefin elimination) transformation of dialkyl phosphonates to phosphonic acids was demonstrated by Han and Coll. [86]. Different synthetic approaches to the hydrolysis of phosphinates and phosphonates have been reviewed very recently [87], and nothing prevents researchers from using these methods in the synthesis of PVPA and other sidechain PCPAs.
2.3.2. Copolymerization of VPA and VPA Derivatives with Other Vinyl Monomers
Since VPA homopolymers have the prospective but limited potential of applications, valuable works on the synthesis of copolymers containing –CH2CHP(O)(OH)2– fragments were published even at the end of the last century.
The free-radical copolymerization of acrylic acid and VPA was conducted by Budd and Coll. in aqueous media (Scheme 21), AIBA was used as an initiator [88]. As the VPA content in the feed was increased, the monomer conversion and yield of the copolymers showed a general decrease. The reactivity ratios of acrylic acid (r1) and VPA (r2) were 4.09 and 0.042, respectively. Such a high difference in r1 and r2 values may give rise to composition drift, where the composition of the copolymer changes as the polymerization proceeds. Where the VPA content in the feed was below 50%, a chain transfer agent was introduced into the polymerization to restrict the molecular weight. Using this method, a range of copolymer compositions were produced with consistent molecular weights (Mw = 150–200 kDa) up to a VPA content of 59 mol%. At higher VPA contents, high-MW polymers were not obtained, and the highest Mw for VPA homopolymer was 29 kDa.
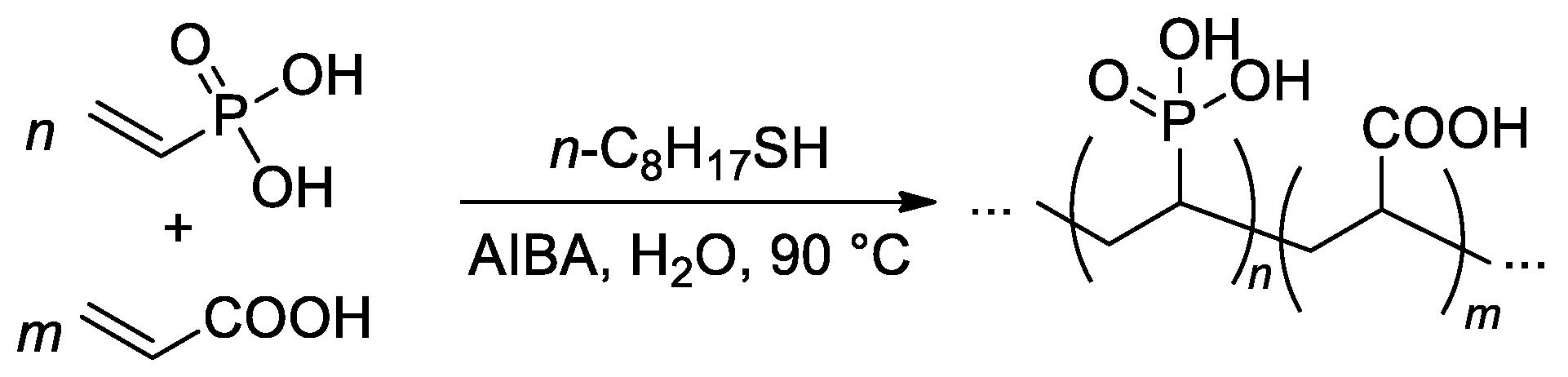
Scheme 21. Free-radical copolymerization of acrylic acid and VPA [88].
RAFT/MADIX approach was used in the synthesis of poly(acrylamide)-b-PVPA copolymers (Scheme 22) [89] with a given length of poly(acrylamide) block and varied length of PVPA fragment, copolymers with Mn 5.5–11.4 kDa were obtained.
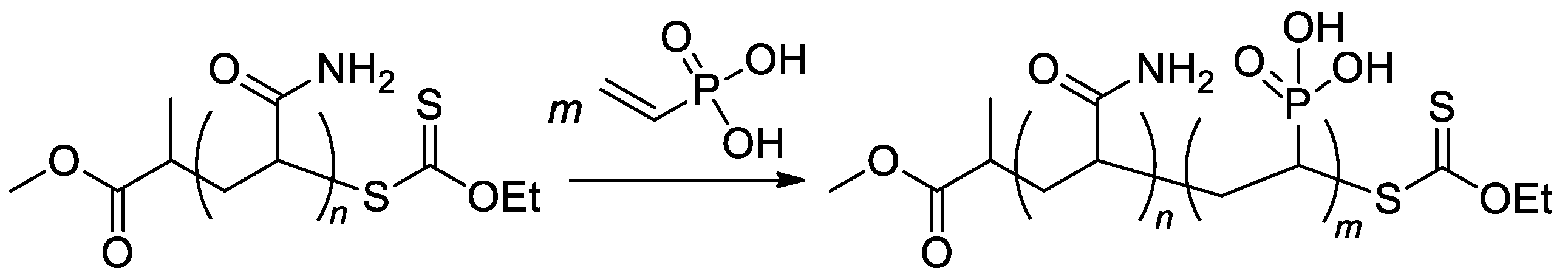
Scheme 22. Aqueous RAFT/MADIX polymerization of VPA via chain extension of a polyacrylamide macroxanthate [89].
When using ethylene glycol diacrylate, cross-linked copolymers of acrylic acid and VPA were obtained [90]. Copolymers of VPA and 1-vinyl-1,2,4-triazole with 1:1, 1:2, and 2:1 comonomer ratios were obtained in DMF solution at 85 °C with AIBN initiator, the Mn values ranged from 6.0 to 8.2 kDa, the dispersity ÐM was ~2 [91]. Copolymerization of VPA and methacrylic ester of mPEG 475 was carried out in ethyl acetate with the use of AIBN as an initiator, side reactions and gel formation were detected [92].
UV-initiated copolymerization of VPA and acrylamide at the oxidized silicon surface in the presence of 2,2-dimethoxy-2-phenylacetophenone (DMPA) was described in [93], the binding of the copolymer with the surface was confirmed by photoelectron spectroscopy.
The synthesis of CH2=CHP(O)(OMe)2, CH2=CF2, and CH2=CHSi(OEt)3 terpolymers was described in [94]. The best results were obtained when using 2,5-dimethyl-2,5-di(tert-butylperoxy)hexane as an initiator of the reaction that was conducted in dimethyl carbonate at 115 °C. Si–O–Si cross-linking was accompanied by partial hydrolysis of methyl phosphonate fragments.
Poly(styrene)-b-PVPA was obtained by nBuLi-initiated sequential anionic polymerization of styrene and CH2=CHP(O)(OiPr)2 in THF media with subsequent hydrolysis [68].
The syntheses of VPA copolymers with 2-deoxy-2-methacrylamido-D-glucose, 4-acryloylmorpholine, or acrylamide, were the subject of the recent study of Nazarova and Coll. [95]. The reactions were conducted in DMF or MeOH (AIBN initiator) or aqueous media (AIBA initiator). VPA has demonstrated values of reactivity comparable with 4-acryloylmorpholine and acrylamide, thus confirming the preference for the use of VPA instead of VPA esters in copolymerization.
With the use of (NH4)2S2O8 initiated free-radical polymerization in aqueous media, random terpolymers of VPA, acrylonitrile, and methyl acrylate were synthesized [96]. Poly(vinylphosphonic acid-co-styrene-co-maleic anhydride) was obtained using AIBN as an initiator and DMSO as a solvent [97]. Water-soluble initiator was used in the random copolymerization of VPA with 5-(methacryloylamido)tetrazole [98].
As a final note, the use of rare-earth metal complexes in the synthesis of copolymers of vinyl phosphonates was the subject of the recent review of Rieger et al. [81].
2.3.3. Homopolymerization and Copolymerization of Phosphorylated Acrylates
Derivatives of acrylic and methacrylic acid, containing –OP(O)(OH)2 and –P(O)(OH)2 fragments, have been evidently regarded as prospective monomers for the synthesis of sidechain PCPAs. There is extensive literature on this subject, resesarchers have tried to present the most interesting and recent works in this field.
Evidently, one of the common methods of the polymerization of acrylates—anionic polymerization—is not applicable for derivatives of phosphoric and phosphinic acids, and the vast majority of research has used free-radical polymerization in one way or another. Trivial methods of free-radical polymerization (solution process, azo compounds, peroxides or UV exposure as initiators, no CTAs or other additives) are fully applicable to phosphorylated and phosphonated acrylates. This is particularly true in the case of acrylates, containing the phosphorus atom in an alkoxy fragment of the acrylate molecule (Scheme 4), due to the high reactivity of these monomers, and a number of examples of the homo- and co-polymerization of phosphorylated and phosphonated (meth)acrylates can be cited [15][16][18][19][21][23][24][30][31][32][99][100][101]. Note that the studies of AIBN-initiated copolymerization of methyl methacrylate and CH2=C(Me)C(O)OCH2P(O)(OMe)OH showed r1 and r2 values of 0.98 and 1.03, respectively, thus confirming close reactivity of ‘conventional’ and phosphonated acrylates [23].
On the other hand, RAFT homo- and co-polymerization were successfully applied to similar acrylate monomers [22][26][102]. In particular, zwitterionic diblock copolymers were synthesized by copolymerization of the macro-RAFT agent, PMEMA (Scheme 23) with methacrylate monomers MOEP and MMPN via RAFT polymerization [22].
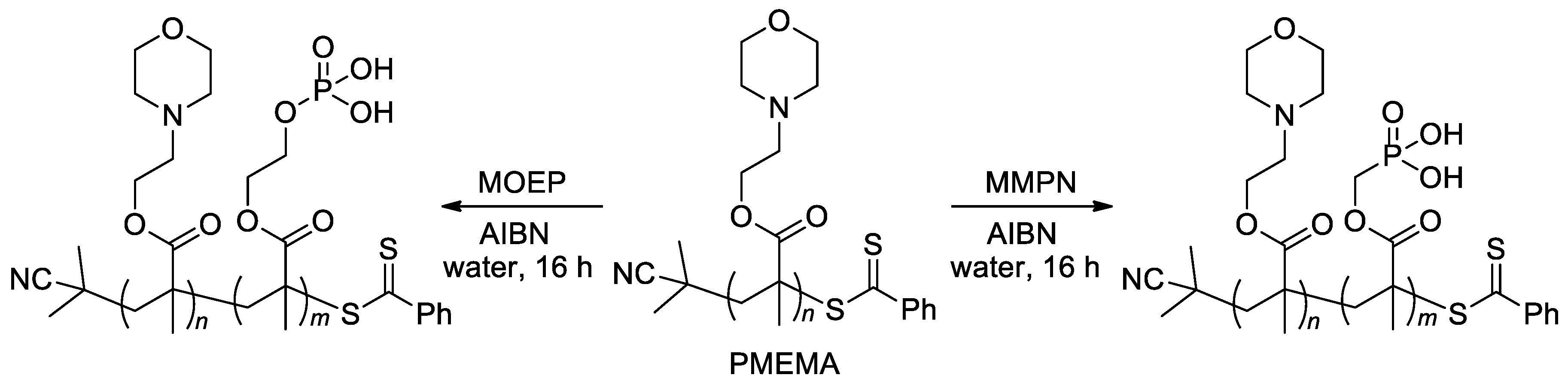
Scheme 23. Synthesis of diblock copolymers via RAFT polymerization [22].
Copolymers of MPC and methyl acrylate with (4-formylphenyl) fragment via –(OCH2CH2)9– spacer, were synthesized by RAFT polymerization [103]. AIBN-initiated copolymerization of MPC, n-butyl methacrylate, MADP (optionally) and rhodamine-linked methacrylate (optionally, for the pharmacokinetic studies) resulted in copolymers with Mn = 14–43 kDa and ÐM ~2 [33] (Scheme 24).
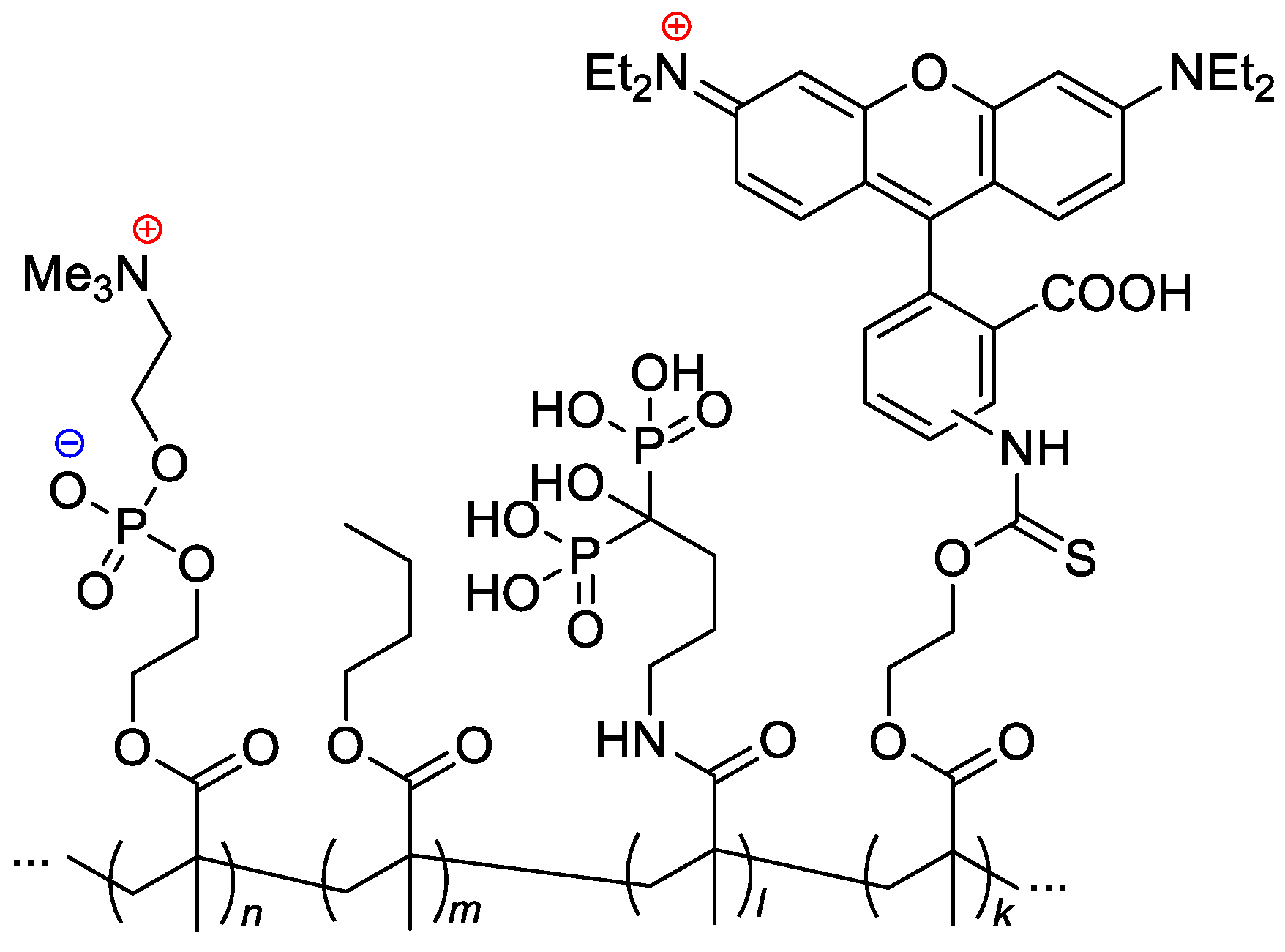
Scheme 24. Formulation of the bone-targeting phospholipid copolymers [33].
The hyper-crosslinked polymer was obtained by AIBN-initiated free-radical polymerization of (CH2=C(Me)C(O)OCH2CH2O)2P(O)OH (BMEP) in DMF [104].
The zwitterionic block copolymer was synthesized by sequential carrying out of organocatalytic ROP of 2-isopropoxy-1,3,2-dioxaphospholane 2-oxide (iPrOEP) with the use of BrCMe2C(O)O(CH2)2OH as an initiator, and atom transfer radical polymerization (ATRP) of the phosphorylcholine-substituted methacrylate (MPC) in the presence of CuBr and 2,2′-bipyridine (Scheme 25) [105]. Note that MOEP-based polymer brushes were obtained with the use of the ATRP approach, based on an SH-substituted –CMe2Br initiator bonded with gold nanoparticles [106].
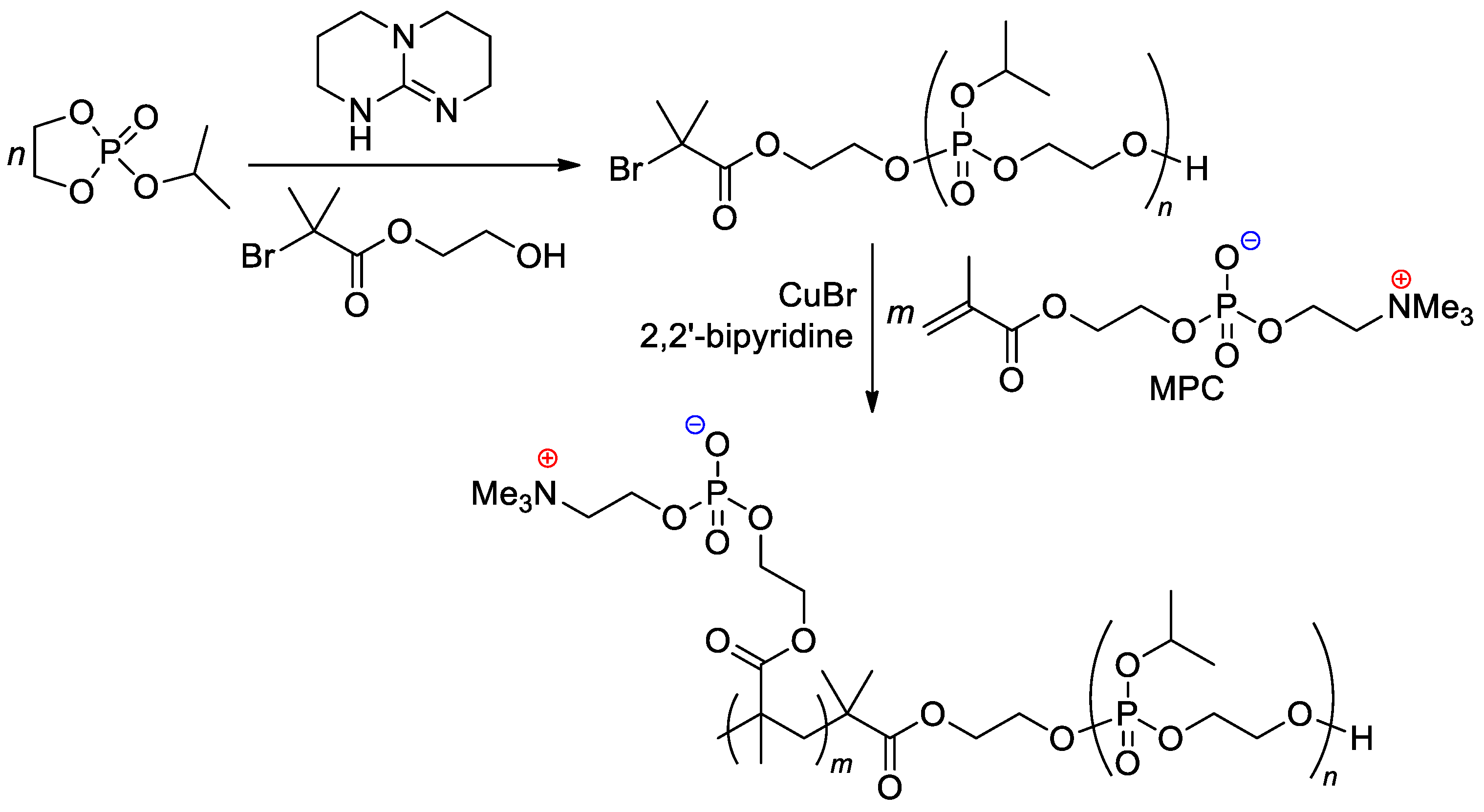
Scheme 25. Synthetic route to block copolymer containing both main-chain and sidechain phosphate fragments [105].
The synthesis of the diblock copolymer, containing mPEG-5000 fragment and polymer of (2-((2-(ethoxycarbonyl)allyl)oxy)ethyl)phosphonic acid as the second block [107], is of particular interest due to the chosen method, ATRP with thiol CTA and macroinitiator PEGA2000 (Scheme 26).
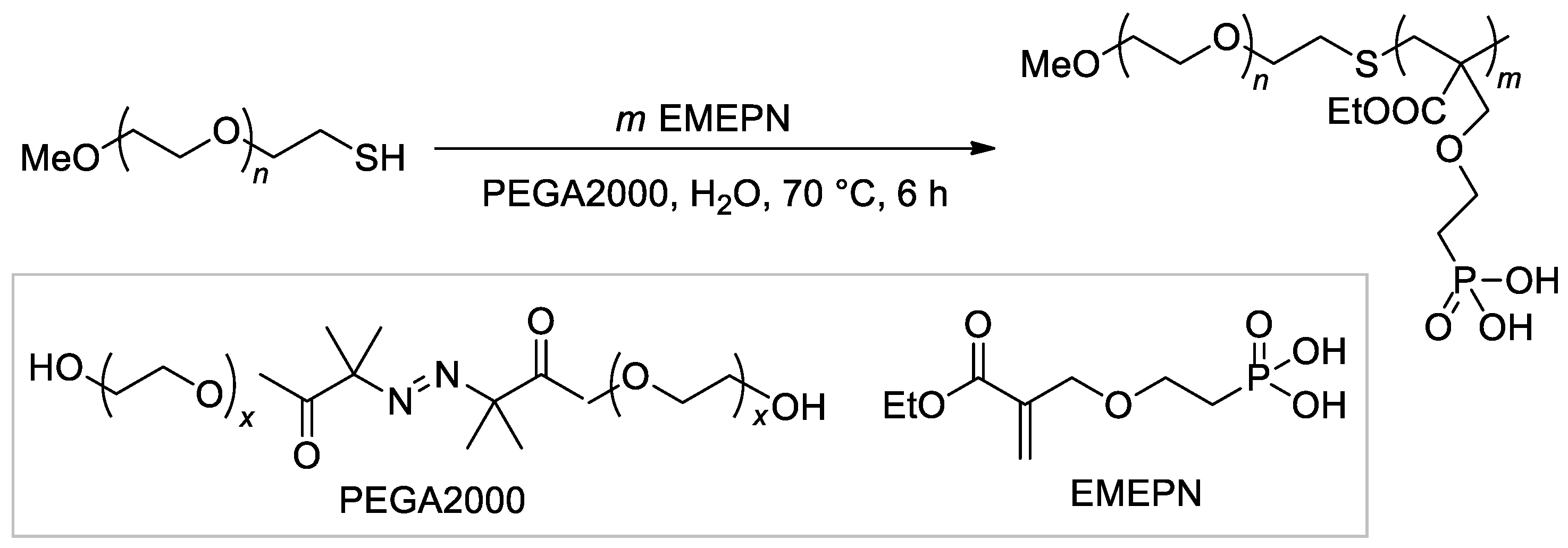
Scheme 26. The use of ATRP with PEGA2000 initiator in the synnthesis of hydrophilic diblock-copolymer [107].
2.3.4. Homopolymerization and Copolymerization of Other Vinyl Monomers
Copolymers of CF2=CF2, CF2=CF(C3F7) and CF2=CFO(CF2)3P(O)(OMe)2 were obtained with the use of AIBN-initiated reaction in 1,1,2-trichloro-1,2,2-trifuoroethane (R-113) media [41]. These copolymers as such have not demonstrated outstanding characteristics, but, nevertheless, a very significant chemical aspect should be mentioned here. Whereas hydrolysis of –P(O)(OR)2-functionalized polymers is not accompanied by noteworthy side reactions, hydrolytic cleavage of the –CF2P(O)(OMe)2 fragment is not so straightforward (Scheme 27). Under basic conditions, the cleavage of the C–P bond was observed, but acidic hydrolysis resulted in the formation of the phosphinic acid.
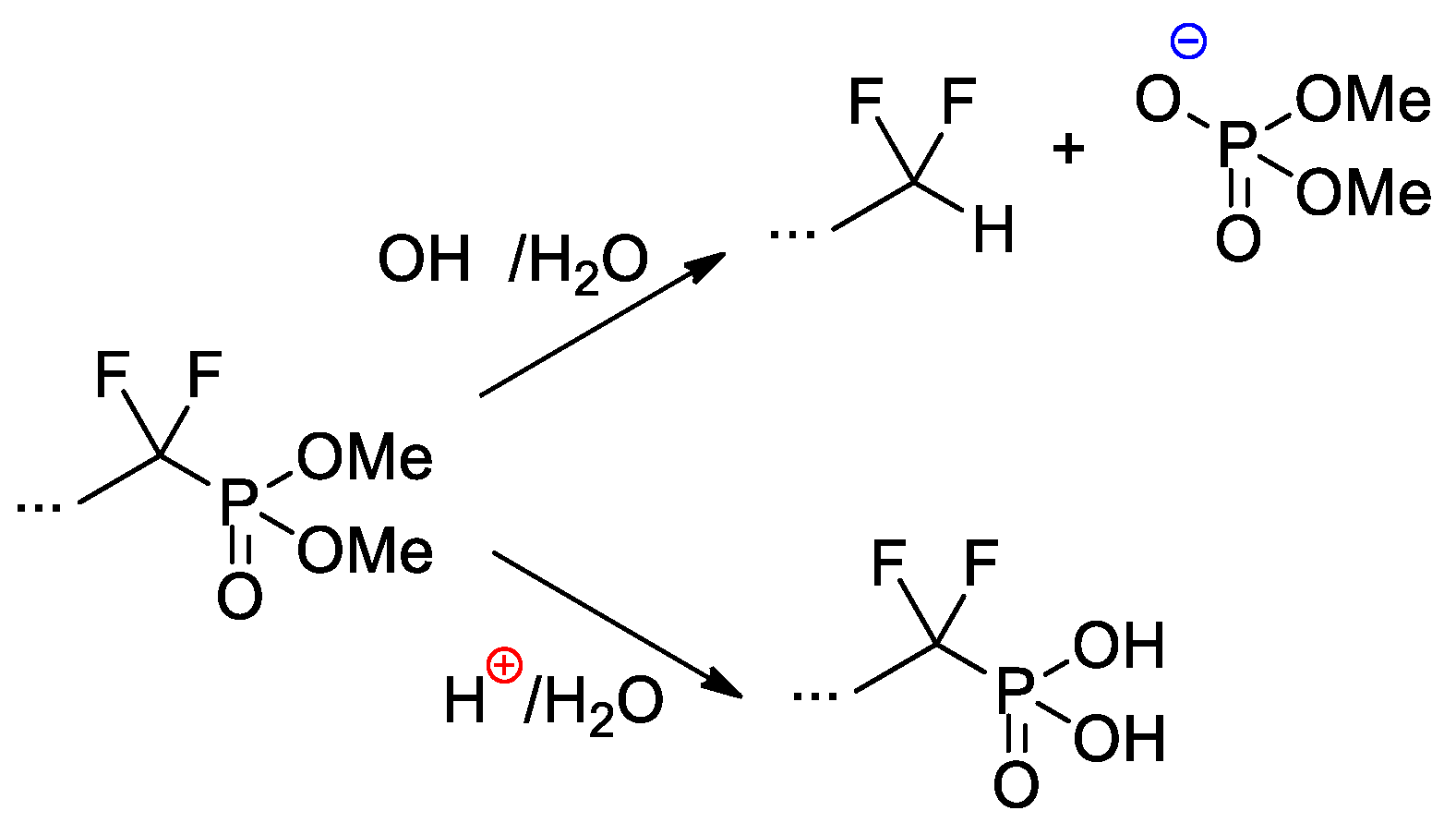
Scheme 27. Hydrolysis of perfluoroalkyl phosphonate fragment [41].
For the synthesis of homopolymers of phosphonated styrenes SP and SbP (Scheme 9) and their block copolymers with poly(isobutylene) Park and Coll. used ATRP in toluene media with CuCl/N,N,N′,N″,N″-pentamethyldiethylenetriamine catalytic system [44]. Corresponding PCPAs were obtained by treatment with Me3SiBr/CHCl3 (36 h at 40 °C) followed by 8 h of methanolysis. Alter and Hoge [43] have synthesized –CH2CF2P(O)(OH)2-functionalized polystyrene (Mn = 12.1 kDa, ÐM = 2.42) by AIBN-initiated polymerization of styrene containing—CH2CF2P(O)(OEt)2 substituent in the position 4, followed by two-stage deprotection (reaction with Me3SiBr/CH2Cl2 and methanolysis).
2.3.5. Cyclopolymerization
Starting from diallylamine, both linear and cross-linked PCPAs were synthesized by Al Hamouz and Ali [108] by free-radical diene cyclopolymerization approach (Scheme 28).
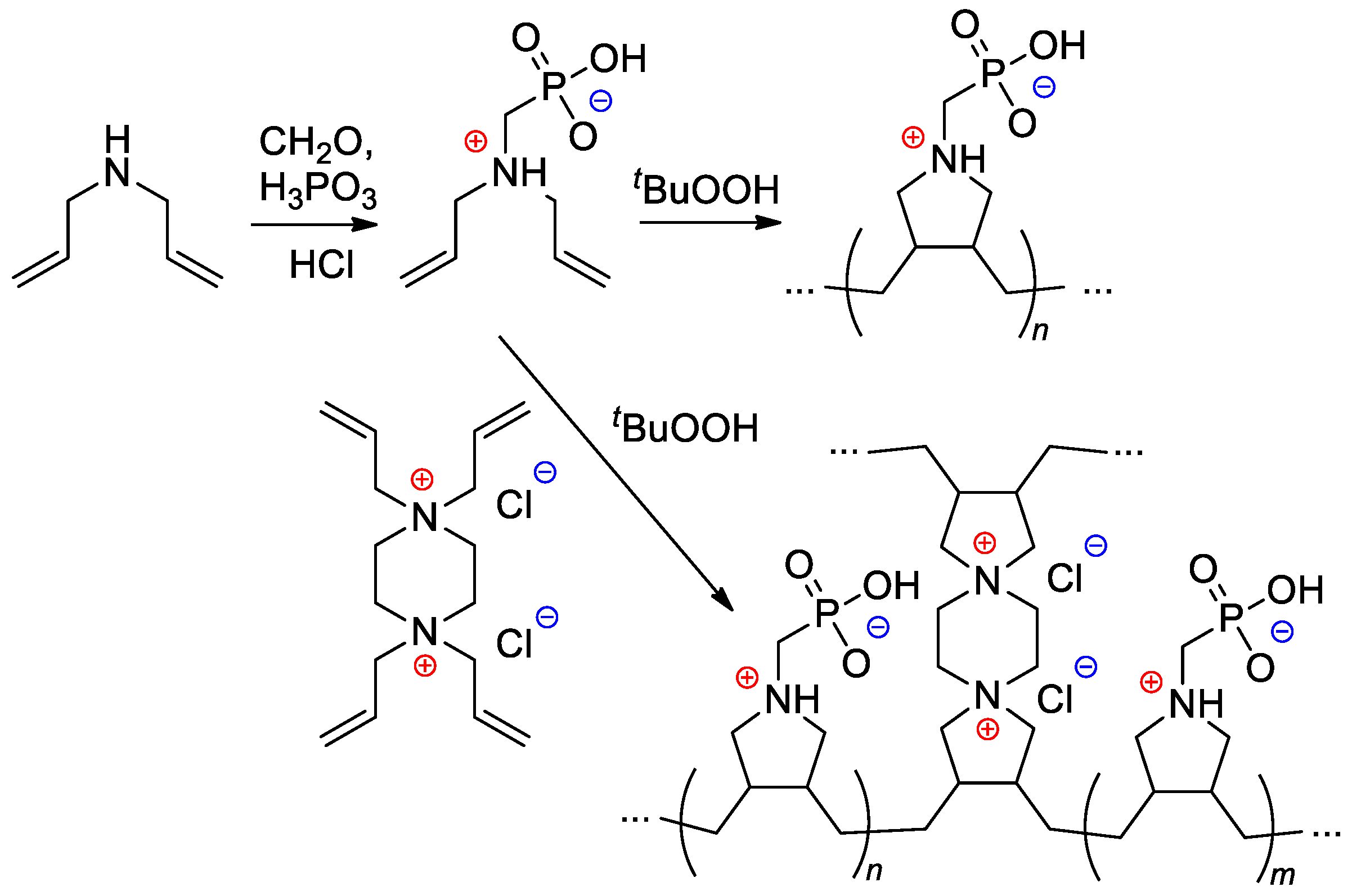
Scheme 28. Synthesis of phosphonate-containing diene monomer and its free-radical diene cyclopolymerization [108].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24021613
References
- Muthukumar, M. 50th Anniversary Perspective: A Perspective on Polyelectrolyte Solutions. Macromolecules 2017, 50, 9528–9560.
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. 2017, 22, 1697–1706.
- Durmaz, E.N.; Sahin, S.; Virga, E.; de Beer, S.; de Smet, L.C.P.M.; de Vos, W.M. Polyelectrolytes as Building Blocks for Next-Generation Membranes with Advanced Functionalities. ACS Appl. Polym. Mater. 2021, 3, 4347–4374.
- Macarie, L.; Ilia, G. Poly(vinylphosphonic acid) and its derivatives. Prog. Polym. Sci. 2010, 35, 1078–1092.
- David, G.; Negrell-Guirao, C.; Iftene, F.; Boutevin, B.; Chougrani, K. Recent progress on phosphonate vinyl monomers and polymers therefore obtained by radical (co)polymerization. Polym. Chem. 2012, 3, 265–274.
- Wehbi, M.; Mehdi, A.; Negrell, C.; David, G.; Alaaeddine, A.; Améduri, B. Phosphorus-Containing Fluoropolymers: State of the Art and Applications. ACS Appl. Mater. Interfaces 2020, 12, 38–59.
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel–dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910.
- Kabachnik, M.I.; Medved, T.Y. Vinylphosphonic acid and some of its derivatives. Russ. Chem. Bull. 1959, 8, 2043–2045.
- Rochlitz, F.; Vilcsek, H. β-Chloroethylphosphonic Dichloride: Its Synthesis and Use. Angew. Chem. Int. Ed. 1962, 1, 652–656.
- Kleiner, H.-J.; Dürsch, W. Process for the Preparation of Vinylphosphonic Acid Diesters and Vinylphosphonic Acid. U.S. Patent 4493803, 15 January 1985. Available online: https://patents.google.com/patent/US4493803A/en?oq=US4493803+(A) (accessed on 20 November 2022).
- Kleiner, H.-J.; Roscher, G. Process for Preparing Vinyl-Phosphonic Acids. U.S. Patent 5811575, 22 September 1998. Available online: https://patents.google.com/patent/US5811575A/en?oq=US5811575+(A) (accessed on 20 November 2022).
- Zotov, S.B.; Tuzhikov, M.O.; Tuzhikov, O.I.; Khokhlova, T.V. Microwave synthesis of vinylphosphonic acid and its derivatives. Russ. J. Appl. Chem. 2012, 85, 639–643.
- Svara, J.; Weferling, N.; Hofmann, T. Phosphorus Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry, 2nd ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2006.
- Becker, L.W. Poly (Alkenyl) Phosphonic Acid and Methods of Use Thereof. U.S. Patent 4446046, 1 May 1984. Available online: https://patents.google.com/patent/US4446046A/en (accessed on 20 November 2022).
- Zeuner, F.; Moszner, N.; Völkel, T.; Vogel, K.; Rheinberger, V. Synthesis and Dental Aspects of Acrylic Phosphoric and Phosphonic Acids. Phosphorus Sulfur Silicon Relat. Elem. 1999, 144, 133–136.
- Song, I.T.; Stewart, R.J. Complex coacervation of Mg(ii) phospho-polymethacrylate, a synthetic analog of sandcastle worm adhesive phosphoproteins. Soft Matter 2018, 14, 379–386.
- Browne, J.E.; Driver, M.J.; Russell, J.C.; Sammes, P.G. Preparation of phospholipid analogues using the phosphoramidite route. J. Chem. Soc. Perkin Trans. 2000, 653–657.
- Avci, D.; Albayrak, A.Z. Synthesis and copolymerization of new phosphorus-containing acrylates. J. Polym. Sci. A Polym. Chem. 2003, 41, 2207–2217.
- Avci, D.; Mathias, L.J. Synthesis and polymerization of phosphorus-containing acrylates. J. Polym. Sci. A Polym. Chem. 2002, 40, 3221–3231.
- Moszner, N.; Zeuner, F.; Fischer, U.K.; Rheinberger, V. Monomers for adhesive polymers, 2. Synthesis and radical polymerisation of hydrolytically stable acrylic phosphonic acids. Macromol. Chem. Phys. 1999, 200, 1062–1067.
- Sahin, G.; Avci, D.; Karahan, O.; Moszner, N. Synthesis and photopolymerizations of new phosphonated methacrylates from alkyl α-hydroxymethacrylates and glycidyl methacrylate. J. Appl. Polym. Sci. 2009, 114, 97–106.
- Samav, Y.; Akpinar, B.; Kocak, G.; Bütün, V. Preparation of Responsive Zwitterionic Diblock Copolymers Containing Phosphate and Phosphonate Groups. Macromol. Res. 2020, 28, 1134–1141.
- El Asri, Z.; Chougrani, K.; Negrell-Guirao, C.; David, G.; Boutevin, B.; Loubat, C. An efficient process for synthesizing and hydrolyzing a phosphonated methacrylate: Investigation of the adhesive and anticorrosive properties. J. Polym. Sci. A Polym. Chem. 2008, 46, 4794–4803.
- Bressy-Brondino, C.; Boutevin, B.; Hervaud, Y.; Gaboyard, M. Adhesive and anticorrosive properties of poly(vinylidene fluoride) powders blended with phosphonated copolymers on galvanized steel plates. J. Appl. Polym. Sci. 2002, 83, 2277–2287.
- Catel, Y.; Bock, T.; Moszner, N. Monomers for adhesive polymers. XV. Synthesis, photopolymerization, and adhesive properties of polymerizable β-ketophosphonic acids. J. Polym. Sci. A Polym. Chem. 2014, 52, 3550–3563.
- Suzuki, S.; Whittaker, M.R.; Grøndahl, L.; Monteiro, M.J.; Wentrup-Byrne, E. Synthesis of Soluble Phosphate Polymers by RAFT and Their in Vitro Mineralization. Biomacromolecules 2006, 7, 3178–3187.
- Catel, Y.; Besse, V.; Zulauf, A.; Marchat, D.; Pfund, E.; Pham, T.-N.; Bernache-Assolant, D.; Degrange, M.; Lequeux, T.; Madec, P.-J.; et al. Synthesis and evaluation of new phosphonic, bisphosphonic and difluoromethylphosphonic acid monomers for dental application. Eur. Polym. J. 2012, 48, 318–330.
- Derbanne, M.; Zulauf, A.; Le Goff, S.; Pfund, E.; Sadoun, M.; Pham, T.-N.; Lequeux, T. Fluorophosphonylated Monomers for Dental Applications. Org. Process Res. Dev. 2014, 18, 1010–1019.
- Banerjee, S.; Wehbi, M.; Manseri, A.; Mehdi, A.; Alaaeddine, A.; Hachem, A.; Ameduri, B. Poly(vinylidene fluoride) Containing Phosphonic Acid as Anticorrosion Coating for Steel. ACS Appl. Mater. Interfaces 2017, 9, 6433–6443.
- Catel, Y.; Degrange, M.; Le Pluart, L.; Madec, P.-J.; Pham, T.-N.; Picton, L. Synthesis, photopolymerization and adhesive properties of new hydrolytically stable phosphonic acids for dental applications. J. Polym. Sci. A Polym. Chem. 2008, 46, 7074–7090.
- Salman, S.; Albayrak, A.Z.; Avci, D.; Aviyente, V. Synthesis and modeling of new phosphorus-containing acrylates. J. Polym. Sci. A Polym. Chem. 2005, 43, 2574–2583.
- Moszner, N.; Zeuner, F.; Pfeiffer, S.; Schurte, I.; Rheinberger, V.; Drache, M. Monomers for Adhesive Polymers, 3. Synthesis, Radical Polymerization and Adhesive Properties of Hydrolytically Stable Phosphonic Acid Monomers. Macromol. Mater. Eng. 2001, 286, 225–231.
- Otaka, A.; Yamaguchi, T.; Saisho, R.; Hiraga, T.; Iwasaki, Y. Bone-targeting phospholipid polymers to solubilize the lipophilic anticancer drug. J. Biomater. Mater. Res. 2020, 108, 2090–2099.
- Moszner, N.; Pavlinec, J.; Lamparth, I.; Zeuner, F.; Angermann, J. Monomers for Adhesive Polymers, 6. Synthesis and Radical Polymerisation of 1,3-Bis(methacrylamido)propane-2-yl Dihydrogen Phosphate. Macromol. Rapid Commun. 2006, 27, 1115–1120.
- Pavlinec, J.; Zeuner, F.; Angermann, J.; Moszner, N. Monomers for Adhesive Polymers, 5. Macromol. Chem. Phys. 2005, 206, 1878–1886.
- Sibold, N.; Madec, P.J.; Masson, S.; Pham, T.N. Synthesis and characterization of (co)polymers containing a phosphonate function for use in dental composites. Polymer 2002, 43, 7257–7267.
- Fukuzaki, N.; Nakabayashi, K.; Nakazawa, S.; Murata, S.; Higashihara, T.; Ueda, M. Highly phosphonated poly(N-phenylacrylamide) for proton exchange membranes. J. Polym. Sci. A Polym. Chem. 2011, 49, 93–100.
- Higashihara, T.; Fukuzaki, N.; Tamura, Y.; Rho, Y.; Nakabayashi, K.; Nakazawa, S.; Murata, S.; Ree, M.; Ueda, M. Polymer electrolyte membrane based on polyacrylate with phosphonic acid via long alkyl side chains. J. Mater. Chem. A 2013, 1, 1457–1464.
- Avci, D.; Mathias, L.J. Synthesis and photopolymerizations of phosphate-containing acrylate/(di)methacrylate monomers from 3-(acryloyloxy)-2-hydroxypropyl methacrylate. Polym. Bull. 2005, 54, 11–19.
- Yeniad, B.; Albayrak, A.Z.; Olcum, N.C.; Avci, D. Synthesis and photopolymerizations of new phosphonated monomers for dental applications. J. Polym. Sci. A Polym. Chem. 2008, 46, 2290–2299.
- Yamabe, M.; Akiyama, K.; Akatsuka, Y.; Kato, M. Novel phosphonated perfluorocarbon polymers. Eur. Polym. J. 2000, 36, 1035–1041.
- Lee, S.-I.; Yoon, K.-H.; Song, M.; Peng, H.; Page, K.A.; Soles, C.L.; Yoon, D.Y. Structure and Properties of Polymer Electrolyte Membranes Containing Phosphonic Acids for Anhydrous Fuel Cells. Chem. Mater. 2012, 24, 115–122.
- Alter, C.; Hoge, B. Synthesis and characterization of a novel difluoromethylene phosphonic acid functionalized polymer. J. Appl. Polym. Sci. 2018, 135, 46765.
- Jang, S.; Kim, S.Y.; Jung, H.Y.; Park, M.J. Phosphonated Polymers with Fine-Tuned Ion Clustering Behavior: Toward Efficient Proton Conductors. Macromolecules 2018, 51, 1120–1128.
- Alter, C.; Neumann, B.; Stammler, H.-G.; Hoge, B. Synthesis and characterization of a novel highly phosphonated water-insoluble polymer. J. Appl. Polym. Sci. 2020, 137, 48235.
- Funk, R.L.; Stallman, J.B.; Wos, J.A. Claisen rearrangements of enol phosphates. J. Am. Chem. Soc. 1993, 115, 8847–8848.
- Nicolaou, K.C.; Yu, R.; Shi, L.; Cai, Q.; Lu, M.; Heretsch, P. General Synthetic Approach to Functionalized Dihydrooxepines. Org. Lett. 2013, 15, 1994–1997.
- Jackson, J.A.; Hammond, G.B.; Wiemer, D.F. Synthesis of .alpha.-phosphono lactones and esters through a vinyl phosphate-phosphonate rearrangement. J. Org. Chem. 1989, 54, 4750–4754.
- Lee, K.; Jackson, J.A.; Wiemer, D.F. Stereocontrol in Horner-Wadsworth-Emmons condensations of α-phosphono lactones with aldehydes: A synthesis of integerrinecic acid and senecic acid lactones. J. Org. Chem. 1993, 58, 5967–5971.
- Bingöl, B.; Meyer, W.H.; Wagner, M.; Wegner, G. Synthesis, Microstructure, and Acidity of Poly(vinylphosphonic acid). Macromol. Rapid Commun. 2006, 27, 1719–1724.
- Komber, H.; Steinert, V.; Voit, B. 1H, 13C, and 31P NMR Study on Poly(vinylphosphonic acid) and Its Dimethyl Ester. Macromolecules 2008, 41, 2119–2125.
- Millaruelo, M.; Steinert, V.; Komber, H.; Klopsch, R.; Voit, B. Synthesis of Vinylphosphonic Acid Anhydrides and their Copolymerization with Vinylphosphonic Acid. Macromol. Chem. Phys. 2008, 209, 366–374.
- Kim, Y.K.; Gu, L.; Bryan, T.E.; Kim, J.R.; Chen, L.; Liu, Y.; Yoon, J.C.; Breschi, L.; Pashley, D.H.; Tay, F.R. Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials 2010, 31, 6618–6627.
- Tutgun, M.S.; Sinirlioglu, D.; Celik, S.U.; Bozkurt, A. Effect of hexagonal boron nitride on poly(vinyl phosphonic acid) composite polymer electrolytes for fuel cells. Polym. Sci. A 2016, 58, 810–817.
- Asian, A.; Bozkurt, A. Nanocomposite polymer electrolyte membranes based on poly(vinylphosphonic acid)/TiO2 nanoparticles. J. Mater. Res. 2012, 27, 3090–3095.
- Durmus, Z.; Kavas, H.; Sozeri, H.; Toprak, M.S.; Aslan, A.; Baykal, A. Poly(vinyl phosphonic acid) (PVPA)–BaFe12O19 Nanocomposite. J. Supercond. Nov. Magn. 2012, 25, 1185–1193.
- David, G.; Boutevin, B.; Seabrook, S.; Destarac, M.; Woodward, G.; Otter, G. Radical Telomerisation of Vinyl Phosphonic Acid with a Series of Chain Transfer Agents. Macromol. Chem. Phys. 2007, 208, 635–642.
- Taherkhani, Z.; Abdollahi, M.; Sharif, A. Synthesis and microstructural characterization of low to high molecular weight poly(vinylphosphonic acid)s: Effect of molecular weight and temperature on acidity and polyelectrolyte behavior. J. Polym. Res. 2017, 24, 132.
- David, G.; Boyer, C.; Tayouo, R.; Seabrook, S.; Ameduri, B.; Boutevin, B.; Woodward, G.; Destarac, M. A Process for Polymerizing Vinyl Phosphonic Acid with C6F13I Perfluoroalkyl Iodide Chain-Transfer Agent. Macromol. Chem. Phys. 2008, 209, 75–83.
- Blidi, I.; Geagea, R.; Coutelier, O.; Mazières, S.; Violleau, F.; Destarac, M. Aqueous RAFT/MADIX polymerisation of vinylphosphonic acid. Polym. Chem. 2012, 4, 609–612.
- Seiler, L.; Loiseau, J.; Leising, F.; Boustingorry, P.; Harrisson, S.; Destarac, M. Acceleration and improved control of aqueous RAFT/MADIX polymerization of vinylphosphonic acid in the presence of alkali hydroxides. Polym. Chem. 2017, 8, 3825–3832.
- Ellis, J.; Wilson, A.D. Polyphosphonate cements: A new class of dental materials. J. Mater. Sci. Lett. 1990, 9, 1058–1060.
- Braybrook, J.H.; Nicholson, J.W. Incorporation of crosslinking agents into poly(vinyl phosphonic acid) as a route to glass–polyalkenoate cements of improved compressive strength. J. Mater. Chem. 1993, 3, 361–365.
- Jin, S.; Gonsalves, K.E. Synthesis and Characterization of Functionalized Poly(ε-caprolactone) Copolymers by Free-Radical Polymerization. Macromolecules 1998, 31, 1010–1015.
- Bingöl, B.; Hart-Smith, G.; Barner-Kowollik, C.; Wegner, G. Characterization of Oligo(vinyl phosphonate)s by High-Resolution Electrospray Ionization Mass Spectrometry: Implications for the Mechanism of Polymerization. Macromolecules 2008, 41, 1634–1639.
- Macarie, L.; Pekar, M.; Simulescu, V.; Plesu, N.; Iliescu, S.; Ilia, G.; Tara-Lunga-Mihali, M. Properties in aqueous solution of homo- and copolymers of vinylphosphonic acid derivatives obtained by UV-curing. Macromol. Res. 2017, 25, 214–221.
- Yin, M.; Kang, N.; Cui, G.; Liu, Z.; Wang, F.; Yang, W.; Klapper, M.; Müllen, K. Synthesis, Electrochemical Properties and Self-Assembly of a Proton-Conducting Core–Shell Macromolecule. Chem. Eur. J. 2012, 18, 2239–2243.
- Wagner, T.; Manhart, A.; Deniz, N.; Kaltbeitzel, A.; Wagner, M.; Brunklaus, G.; Meyer, W.H. Vinylphosphonic Acid Homo- and Block Copolymers. Macromol. Chem. Phys. 2009, 210, 1903–1914.
- Rabinowitz, R. The Reactions of Phosphonic Acid Esters with Acid Chlorides. A Very Mild Hydrolytic Route. J. Org. Chem. 1963, 28, 2975–2978.
- Kawauchi, T.; Ohara, M.; Udo, M.; Kawauchi, M.; Takeichi, T. Preparation of isotactic-rich poly(dimethyl vinylphosphonate) and poly(vinylphosphonic acid) via the anionic polymerization of dimethyl vinylphosphonate. J. Polym. Sci. A Polym. Chem. 2010, 48, 1677–1682.
- Rabe, G.W.; Komber, H.; Häussler, L.; Kreger, K.; Lattermann, G. Polymerization of Diethyl Vinylphosphonate Mediated by Rare-Earth Tris(amide) Compounds. Macromolecules 2010, 43, 1178–1181.
- Seemann, U.B.; Dengler, J.E.; Rieger, B. High-Molecular-Weight Poly(vinylphosphonate)s by Single-Component Living Polymerization Initiated by Rare-Earth-Metal Complexes. Angew. Chem. Int. Ed. 2010, 49, 3489–3491.
- Salzinger, S.; Seemann, U.B.; Plikhta, A.; Rieger, B. Poly(vinylphosphonate)s Synthesized by Trivalent Cyclopentadienyl Lanthanide-Induced Group Transfer Polymerization. Macromolecules 2011, 44, 5920–5927.
- Zhang, N.; Salzinger, S.; Rieger, B. Poly(vinylphosphonate)s with Widely Tunable LCST: A Promising Alternative to Conventional Thermoresponsive Polymers. Macromolecules 2012, 45, 9751–9758.
- Salzinger, S.; Soller, B.S.; Plikhta, A.; Seemann, U.B.; Herdtweck, E.; Rieger, B. Mechanistic Studies on Initiation and Propagation of Rare Earth Metal-Mediated Group Transfer Polymerization of Vinylphosphonates. J. Am. Chem. Soc. 2013, 135, 13030–13040.
- Soller, B.S.; Salzinger, S.; Jandl, C.; Pöthig, A.; Rieger, B. C–H Bond Activation by σ-Bond Metathesis as a Versatile Route toward Highly Efficient Initiators for the Catalytic Precision Polymerization of Polar Monomers. Organometallics 2015, 34, 2703–2706.
- Soller, B.S.; Sun, Q.; Salzinger, S.; Jandl, C.; Pöthig, A.; Rieger, B. Ligand Induced Steric Crowding in Rare Earth Metal-Mediated Group Transfer Polymerization of Vinylphosphonates: Does Enthalpy Matter? Macromolecules 2016, 49, 1582–1589.
- Pahl, P.; Schwarzenböck, C.; Herz, F.A.D.; Soller, B.S.; Jandl, C.; Rieger, B. Core-First Synthesis of Three-Armed Star-Shaped Polymers by Rare Earth Metal-Mediated Group Transfer Polymerization. Macromolecules 2017, 50, 6569–6576.
- Weger, M.; Pahl, P.; Schmidt, F.; Soller, B.S.; Altmann, P.J.; Pöthig, A.; Gemmecker, G.; Eisenreich, W.; Rieger, B. Isospecific Group-Transfer Polymerization of Diethyl Vinylphosphonate and Multidimensional NMR Analysis of the Polymer Microstructure. Macromolecules 2019, 52, 7073–7080.
- Salzinger, S.; Rieger, B. Rare Earth Metal-Mediated Group Transfer Polymerization of Vinylphosphonates. Macromol. Rapid Commun. 2012, 33, 1327–1345.
- Soller, B.S.; Salzinger, S.; Rieger, B. Rare Earth Metal-Mediated Precision Polymerization of Vinylphosphonates and Conjugated Nitrogen-Containing Vinyl Monomers. Chem. Rev. 2016, 116, 1993–2022.
- Zhang, N.; Salzinger, S.; Deubel, F.; Jordan, R.; Rieger, B. Surface-Initiated Group Transfer Polymerization Mediated by Rare Earth Metal Catalysts. J. Am. Chem. Soc. 2012, 134, 7333–7336.
- Yang, J.; Liang, Y.; Salzinger, S.; Zhang, N.; Dong, D.; Rieger, B. Poly(vinylphosphonate)s functionalized polymer microspheres via rare earth metal-mediated group transfer polymerization. J. Polym. Sci. A Polym. Chem. 2014, 52, 2919–2925.
- Altenbuchner, P.T.; Soller, B.S.; Kissling, S.; Bachmann, T.; Kronast, A.; Vagin, S.I.; Rieger, B. Versatile 2-Methoxyethylaminobis(phenolate)yttrium Catalysts: Catalytic Precision Polymerization of Polar Monomers via Rare Earth Metal-Mediated Group Transfer Polymerization. Macromolecules 2014, 47, 7742–7749.
- Li, J.; Ni, X.; Ling, J.; Shen, Z. Syntheses and properties of poly(diethyl vinylphosphonate) initiated by lanthanide tris(borohydride) complexes: Polymerization controllability and mechanism. J. Polym. Sci. A Polym. Chem. 2013, 51, 2409–2415.
- Li, C.; Saga, Y.; Onozawa, S.; Kobayashi, S.; Sato, K.; Fukaya, N.; Han, L.-B. Wet and Dry Processes for the Selective Transformation of Phosphonates to Phosphonic Acids Catalyzed by Brønsted Acids. J. Org. Chem. 2020, 85, 14411–14419.
- Harsági, N.; Keglevich, G. The Hydrolysis of Phosphinates and Phosphonates: A Review. Molecules 2021, 26, 2840.
- Dey, R.E.; Zhong, X.; Youle, P.J.; Wang, Q.G.; Wimpenny, I.; Downes, S.; Hoyland, J.A.; Watts, D.C.; Gough, J.E.; Budd, P.M. Synthesis and Characterization of Poly(vinylphosphonic acid-co-acrylic acid) Copolymers for Application in Bone Tissue Scaffolds. Macromolecules 2016, 49, 2656–2662.
- Layrac, G.; Gérardin, C.; Tichit, D.; Harrisson, S.; Destarac, M. Hybrid polyion complex micelles from poly(vinylphosphonic acid)-based double hydrophilic block copolymers and divalent transition metal ions. Polymer 2015, 72, 292–300.
- Dey, R.E.; Wimpenny, I.; Gough, J.E.; Watts, D.C.; Budd, P.M. Poly(vinylphosphonic acid-co-acrylic acid) hydrogels: The effect of copolymer composition on osteoblast adhesion and proliferation. J. Biomed. Mater. Res. 2018, 106, 255–264.
- Çelik, S.Ü.; Akbey, Ü.; Graf, R.; Bozkurt, A.; Spiess, H.W. Anhydrous proton-conducting properties of triazole–phosphonic acidcopolymers: A combined study with MAS NMR. Phys. Chem. Chem. Phys. 2008, 10, 6058–6066.
- Najafi, V.; Kabiri, K.; Ziaee, F.; Omidian, H.; Zohuriaan-Mehr, M.J.; Bouhendi, H.; Farhadnejad, H. Synthesis and characterization of alcogels based on ethylene glycol methyl ether methacrylate-vinyl phosphonic acid copolymers. J. Polym. Res. 2012, 19, 9866.
- Gemeinhart, R.A.; Bare, C.M.; Haasch, R.T.; Gemeinhart, E.J. Osteoblast-like cell attachment to and calcification of novel phosphonate-containing polymeric substrates. J. Biomed. Mater. Res. A 2006, 78, 433–440.
- Wehbi, M.; Mehdi, A.; Alaaeddine, A.; Jaber, N.; Ameduri, B. Solid–Liquid Europium Ion Extraction via Phosphonic Acid-Functionalized Polyvinylidene Fluoride Siloxanes. Polymers 2020, 12, 1955.
- Nazarova, O.; Chesnokova, E.; Nekrasova, T.; Zolotova, Y.; Dobrodumov, A.; Vlasova, E.; Fischer, A.; Bezrukova, M.; Panarin, E. New Copolymers of Vinylphosphonic Acid with Hydrophilic Monomers and Their Eu3+ Complexes. Polymers 2022, 14, 590.
- Yılmaz, M.; Akar, A.; Köken, N.; Kızılcan, N. Polymers of vinylphosphonic acid, acrylonitrile, and methyl acrylate and their nanofibers. J. Appl. Polym. Sci. 2020, 137, 49023.
- Kavlak, S.; Güner, A.; Rzayev, Z.M.O. Functional terpolymers containing vinylphosphonic acid: The synthesis and characterization of poly(vinylphosphonic acid-co-styrene-co-maleic anhydride). J. Appl. Polym. Sci. 2012, 125, 3617–3629.
- Sinirlioglu, D.; Celik, S.U.; Muftuoglu, A.E.; Bozkurt, A. Proton Conducting Copolymer Electrolytes Based on Vinyl Phosphonic Acid and 5-(Methacrylamido)tetrazole. Macromol. Chem. Phys. 2014, 215, 269–279.
- Lane, D.D.; Kaur, S.; Weerasakare, G.M.; Stewart, R.J. Toughened hydrogels inspired by aquatic caddisworm silk. Soft Matter 2015, 11, 6981–6990.
- Uğur, M.H.; Kayaman-Apohan, N.; Avci, D.; Güngör, A. Phosphoric acid functional UV-cured proton conducting polymer membranes for fuel cells. Ionics 2015, 21, 3097–3107.
- George, K.A.; Wentrup-Byrne, E.; Hill, D.J.T.; Whittaker, A.K. Investigation into the Diffusion of Water into HEMA-co-MOEP Hydrogels. Biomacromolecules 2004, 5, 1194–1199.
- Hajiali, F.; Tajbakhsh, S.; Marić, M. Thermal characteristics and flame retardance behavior of phosphoric acid-containing poly(methacrylates) synthesized by RAFT polymerization. Mater. Today Commun. 2020, 25, 101618.
- Li, L.; Song, Y.; He, J.; Zhang, M.; Liu, J.; Ni, P. Zwitterionic shielded polymeric prodrug with folate-targeting and pH responsiveness for drug delivery. J. Mater. Chem. B 2019, 7, 786–795.
- Di, T.; Tan, D.; Yu, Q.; Lin, J.; Zhu, T.; Li, T.; Li, L. Ultra-High Performance of Hyper-Crosslinked Phosphate-Based Polymer for Uranium and Rare Earth Element Adsorption in Aqueous Solution. Langmuir 2019, 35, 13860–13871.
- Iwasaki, Y.; Yamaguchi, E. Synthesis of well-defined thermoresponsive polyphosphoester macroinitiators using organocatalysts. Macromolecules 2010, 43, 2664–2666.
- Zhou, F.; Huck, W.T.S. Three-stage switching of surface wetting using phosphate-bearing polymer brushes. Chem. Commun. 2005, 5999–6001.
- Wang, T.; Rother, G.; Cölfen, H. A New Method to Purify Highly Phosphonated Block Copolymers and Their Effect on Calcium Carbonate Mineralization. Macromol. Chem. Phys. 2005, 206, 1619–1629.
- Al Hamouz, O.C.S.; Ali, S.A. Removal of heavy metal ions using a novel cross-linked polyzwitterionic phosphonate. Sep. Purif. Technol. 2012, 98, 94–101.
This entry is offline, you can click here to edit this entry!
