Thermophilic anaerobic digestion (TAD) technology has been adopted worldwide mainly due to it being a pathogen-free process in addition to the enhanced biogas yield and short hydraulic retention time (HRT). Taking the high metabolic rate of the thermophilic microbial community with highly efficient enzymatic systems into consideration, thermophiles are being widely explored as efficient inocula for lignocellulosic biomass (LCB) degradation and improved biomethane production. The advantages of TAD over mesophilic anaerobic digestion (MAD), including improved kinetics, efficient degradation of organic matter, and economic and environmental sustainability, make it one of the best strategies to be operated at moderately high temperatures.
- thermophilic inoculum
- biogas
- lignocellulosic biomass
- Anaerobic digestion
1. Development of Robust Microbiome: Selection, Acclimatization, and Bioaugmentation of Inoculum
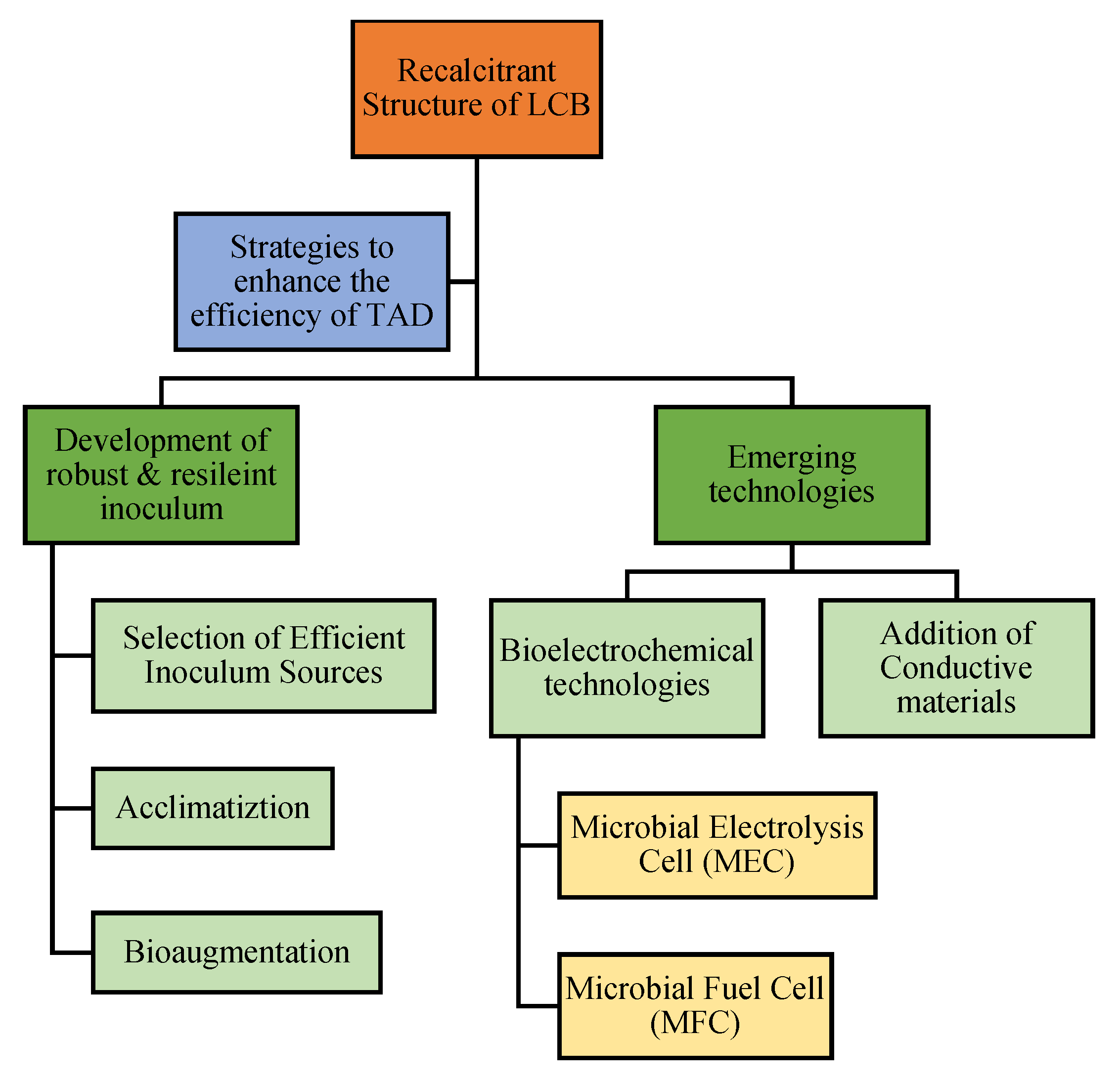
2. Adoption of Emerging Technologies
2.1. Bioelectrochemical Technologies
2.2. Addition of Conductive Materials
This entry is adapted from the peer-reviewed paper 10.3390/su15031859
References
- Fdéz, L.A.; Álvarez-Gallego, C.; Márquez, D.S.; García, L.I.R. Start-up of thermophilic-dry anaerobic digestion of OFMSW using adapted modified SEBAC inoculum. Bioresour. Technol. 2010, 101, 9031–9039.
- Ahring, B.K.; Mladenovska, Z.; Iranpour, R.; Westermann, P. State of the art and future perspectives of thermophilic anaerobic digestion. Water Sci. Technol. 2002, 45, 293–298.
- Bolzonella, D.; Battistoni, P.; Mata-Alvarez, J.; Cecchi, F. Anaerobic digestion of organic solid waste: Process behaviour in transient conditions. Water Sci. Technol. 2003, 48, 1–8.
- Palatsi, J.; Gimenez-Lorang, A.; Ferrer, I.; Flotats, X. Start-up strategies of thermophilic anaerobic digestion of sewage sludge. Water Sci. Technol. 2009, 59, 1777–1784.
- Tian, Z.; Zhang, Y.; Li, Y.; Chi, Y.; Yang, M. Rapid establishment of thermophilic anaerobic microbial community during the one-step startup of thermophilic anaerobic digestion from a mesophilic digester. Water Res. 2015, 69, 9–19.
- Strang, O.; Ács, N.; Wirth, R.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Bioaugmentation of the thermophilic anaerobic biodegradation of cellulose and corn stover. Anaerobe 2017, 46, 104–113.
- Moset, V.; Poulsen, M.; Wahid, R.; Højberg, O.; Møller, H. Mesophilic versus thermophilic anaerobic digestion of cattle manure: Methane productivity and microbial ecology. Microb. Biotechnol. 2015, 8, 787–800.
- Ghanimeh, S.; El-Fadel, M.; Saikaly, P.E. Performance of thermophilic anaerobic digesters using inoculum mixes with enhanced methanogenic diversity. J. Chem. Technol. Biotechnol. 2018, 93, 207–214.
- Cord-ruwisch, R.; Mercz, T.I.; Hoh, C.; Strong, G.E. Dissolved Hydrogen Concentration as an On-Line Control Parameter for the Automated Operation and Optimization of Anaerobic Digesters. Biotechnol. Bioeng. 1997, 56, 626–634.
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Overcoming organic and nitrogen overload in thermophilic anaerobic digestion of pig slurry by coupling a microbial electrolysis cell. Bioresour. Technol. 2016, 216, 362–372.
- Tremouli; Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G. Exploitation of Digestate from Thermophilic and Mesophilic Anaerobic Digesters Fed with Fermentable Food Waste Using the MFC Technology. Waste Biomass Valorization 2021, 12, 5361–5370.
- Carrillo-Peña, D.; Escapa, A.; Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R.; Mateos, R. Bioelectrochemical enhancement of methane production from exhausted vine shoot fermentation broth by integration of MEC with anaerobic digestion. Biomass Convers. Biorefinery 2022, 1–10.
- Park, J.-G.; Lee, B.; Lee, U.-J.; Jun, H.-B. An anaerobic digester with microbial electrolysis cell enhances relative abundance of methylotrophic methanogens in bulk solution. Environ. Eng. Res. 2021, 27, 210666.
- Barua, S.; Zakaria, B.S.; Chung, T.; Hai, F.I.; Haile, T.; Al-Mamun, A.; Dhar, B.R. Microbial electrolysis followed by chemical precipitation for effective nutrients recovery from digested sludge centrate in WWTPs. Chem. Eng. J. 2019, 361, 256–265.
- Barua, S.; Zakaria, B.S.; Al-Mamun, A.; Dhar, B.R. Anodic performance of microbial electrolysis cells in response to ammonia nitrogen. J. Environ. Eng. Sci. 2018, 14, 37–43.
- Zhang, Y.; Angelidaki, I. Submersible microbial desalination cell for simultaneous ammonia recovery and electricity production from anaerobic reactors containing high levels of ammonia. Bioresour. Technol. 2015, 177, 233–239.
- Lu, L.; Ren, Z.J. Microbial electrolysis cells for waste biorefinery: A state of the art review. Bioresour. Technol. 2016, 215, 254–264.
- Yu, Z.; Leng, X.; Zhao, S.; Ji, J.; Zhou, T.; Khan, A. Bioresource Technology A review on the applications of microbial electrolysis cells in anaerobic digestion. ScienceDirect 2017, 255, 340–348.
- Zhang, Q.; Zhao, M.; Wang, T.; Zeng, L.; Bai, C.; Wu, R.; Xing, Z.; Xiao, G.; Shi, X. Enhanced sludge thermophilic anaerobic digestion performance by single-chambered microbial electrolysis cells under ammonia inhibition. J. Environ. Chem. Eng. 2022, 10, 107802.
- Viggi, C.C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543.
- Dubé, C.-D.; Guiot, S.R. Direct Interspecies Electron Transfer in Anaerobic Digestion: A Review. In Biogas Science and Technology, 151st ed.; Gübitz, G., Bauer, A., Bochmann, G., Gronauer, A., Weiss, S., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–200.
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016, 209, 148–156.
- Yan, W.; Shen, N.; Xiao, Y.; Chen, Y.; Sun, F.; Tyagi, V.K.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344.
- Tiwari, S.B.; Dubey, M.; Ahmed, B.; Gahlot, P.; Khan, A.A.; Rajpal, A.; Kazmi, A.; Tyagi, V.K. Carbon-based conductive materials facilitated anaerobic co-digestion of agro waste under thermophilic conditions. Waste Manag. 2021, 124, 17–25.
- Gómez-Quiroga, X.; Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Successful and stable operation of anaerobic thermophilic co-digestion of sun-dried sugar beet pulp and cow manure under short hydraulic retention time. Chemosphere 2022, 293, 133484.
- Cai, M.; Wilkins, D.; Chen, J.; Ng, S.-K.; Lu, H.; Jia, Y.; Lee, P.K.H. Metagenomic reconstruction of key anaerobic digestion pathways in municipal sludge and industrial wastewater biogas-producing systems. Front. Microbiol. 2016, 7, 778.
- Shen, Y.; Forrester, S.; Koval, J.; Urgun-Demirtas, M. Yearlong semi-continuous operation of thermophilic two-stage anaerobic digesters amended with biochar for enhanced biomethane production. J. Clean. Prod. 2017, 167, 863–874.
- Lim, E.Y.; Tian, H.; Chen, Y.; Ni, K.; Zhang, J.; Tong, Y.W. Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresour. Technol. 2020, 314, 123751.
- Dang, Y.; Holmes, D.E.; Zhao, Z.Q.; Woodard, T.L.; Zhang, Y.B.; Sun, D.Z.; Wang, L.Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522.
