Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Flavonoid biosynthesis is responsive to a wide range of stresses, and the numerous synthesized flavonoid species offer two main evolutionary advantages to land plants. First, flavonoids are antioxidants and thus defend plants against those adverse conditions that lead to the overproduction of reactive oxygen species. Second, flavonoids aid in protecting plants against water and nutrient deficiency by modulating root development and establishing symbiotic relations with beneficial soil fungi and bacteria.
- flavonoids
- auxin
- cytokinin
- oxidative stress
- antioxidants
1. Symbiosis with Arbuscular Mycorrhizal Fungi (AMF)
Possibly the best example of flavonoids supporting and modulating the auxin/cytokinin-regulated shoot/root ratio (and, through that, supporting the plant reproduction/well-being balance) are the symbiotic interactions between plants and beneficial bacteria and fungi. The overarching theme is that flavonoids act together with auxin to promote symbiotic interactions, whereas cytokinin—albeit required for some symbiotic development—limits symbiosis to the level adequate for survival, thus ensuring sufficient but not excessive allocation of carbon for this process.
AMF symbiosis was one of the major steps in the evolution of land plants [1]. By interacting with plant roots, the fungal hyphae serve as an extension of the root system, helping plants with water and mineral nutrient uptake [2]. In turn, the fungus receives carbohydrates from the plant, creating a mutually beneficial relationship. AMF symbiosis is widespread and is estimated to involve up to 80% of plant species [3][4].
Both auxin and flavonoids play a positive role in AMF symbiosis [5]. Root-secreted flavonoids have been shown to promote fungal spore germination, hyphal growth, and the fungal colonization of roots [6]. Subsequent research showed a positive correlation between fungal root colonization rates and flavonoid biosynthesis and secretion levels [7]. In addition, fungal inoculation itself leads to the increased synthesis of flavonoids, a process that was shown to be important for successful root colonization and likely based on the flavonoid-dependent inhibition of auxin transport [8]. The fungal interaction also increases auxin synthesis and action, and since this symbiotic interaction is defective in auxin-deficient and auxin-insensitive mutants, this increased auxin action is required for root colonization [9]. Contrary to the positive effects of flavonoid and auxin, cytokinin plays a largely negative role in AMF symbiosis. The establishment of AMF symbiosis leads to increased cytokinin biosynthesis [10], and this is thought to decrease root growth in favor of increased shoot growth, which is a distinctive response to increased nutrient availability and further evidence that cytokinin acts as a satiation hormone [11]. In support of that hypothesis, cytokinin was shown to suppress root penetration and colonization by the fungus [12] as well as hinder carbon-feeding of the fungus by the plant, thus restricting the colonization of roots to levels that combine optimal nutrient uptake with the optimal distribution of carbon between the shoots and roots [13].
AMF symbiosis plays a central role in the acquisition of phosphate from soils [14]. Importantly, soil phosphate content is a major deciding factor in the rate of AMF symbiosis, as high phosphate content tends to suppress this plant–fungal interaction [15]. Phosphate deficiency, on the other hand, leads to a combined increase in auxin action and decreased cytokinin biosynthesis and sensitivity [11], which generates an optimal hormonal balance for the fungal colonization of the roots (Figure 1).
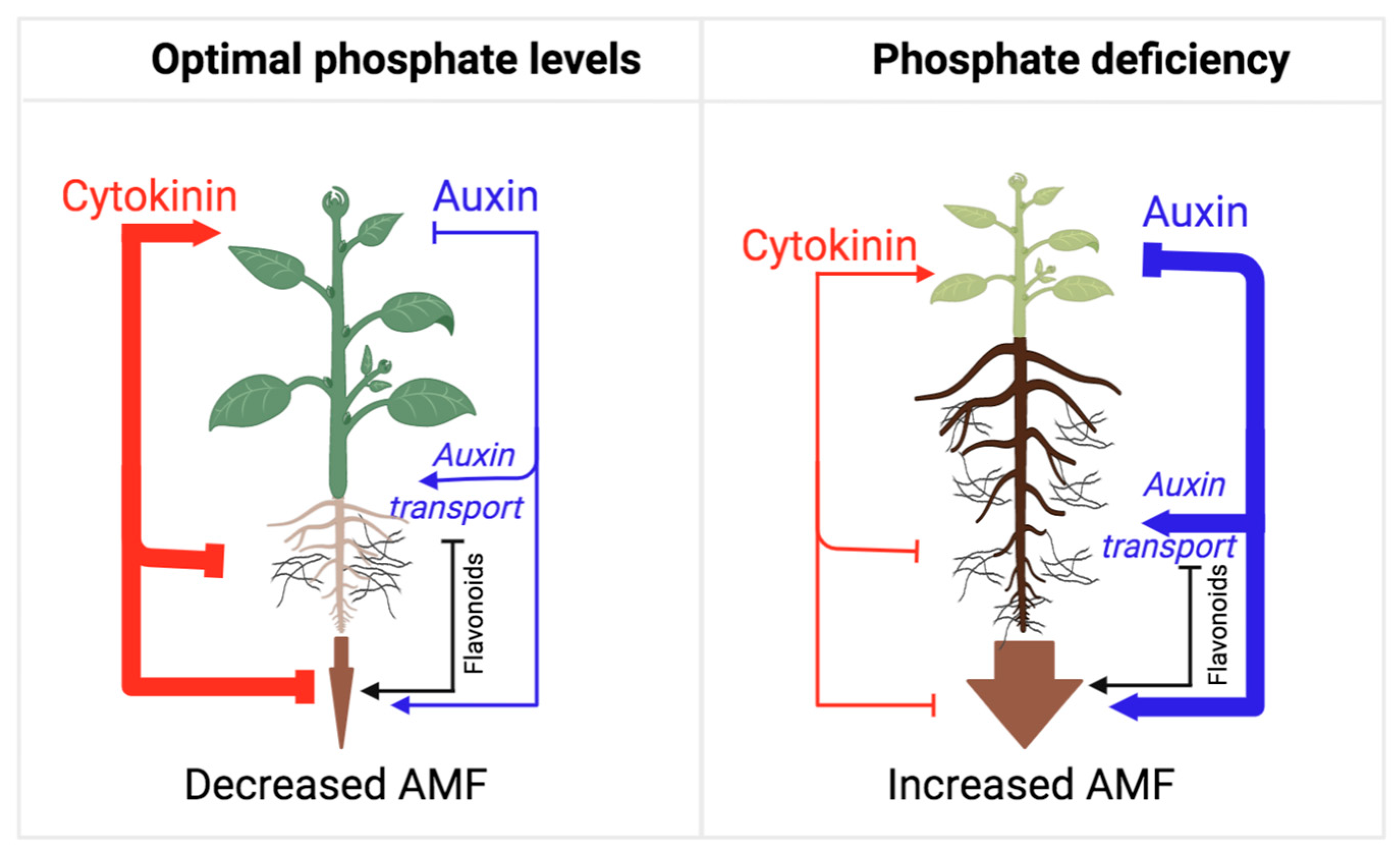
Figure 1. Model illustrating the auxin/cytokinin ratios during the establishment of AMF symbiosis from the perspective of adaptive control of the shoot/root growth ratio. As long as the plant perceives phosphate deficiency in the soil, the rate of AMF symbiosis establishment is high, mirroring a low shoot/root growth ratio and the allocation of resources towards root growth and fungal colonization. Under phosphate-deficient conditions, auxin action is high, which represses cytokinin action and shoot growth in favor of root growth and symbiosis. Under phosphate-sufficient conditions, auxin action is reduced, and cytokinin action is released to promote shoot growth, inhibit root growth, and suppress AMF symbiosis. Flavonoids play an essential role in this process, as they promote several stages of this symbiotic interaction.
2. Rhizobial Symbiosis
Nitrogen availability is a major limiting factor for plant growth, and the ability of some plant species to engage in symbiosis with the nitrogen-fixing bacteria of the Rhizobium genus has provided them with the substantial benefit of having an additional source of ammonium and, thus, nitrogen [16][17][18]. In this symbiotic interaction, the bacteria are housed in nodules, specialized root structures whose initiation and development are triggered by signal exchanges between the plant and bacterium. As with AMF, the Rhizobium symbiont benefits by acquiring photosynthates from the plant.
Flavonoids play multiple roles in Rhizobial symbiosis [19]. While flavonoids’ role as bacterial chemoattractants has been recently questioned [20][21][22], it is well established that, under nitrogen-limiting conditions, legumes secrete flavonoids as root exudates to promote the activation of NodD transcription factors. The activation of NodDs induces the expression of Rhizobia Nod factors, subsequently signaling for the plant to engage in nodule development [19][20][23]. In turn, Rhizobial infection induces flavonoid synthesis, which is important for modulating auxin action in infected roots, further induction of bacterial Nod factor gene expression, and ensuring host-specific bacterial interactions [24][25]. Similarly to AMF symbiosis, auxin plays an overall positive role in Rhizobial symbiosis by promoting nodule formation, a process that involves auxin transport inhibition by flavonols [26]. Cytokinin signaling is also required to establish Rhizobial symbiosis and functions, mainly by promoting the flavonoid biosynthesis needed for auxin transport control [26][27][28]. However, the overall effect of cytokinin appears to be the suppression of nodulation frequency, similar to its satiation hormone activity in AFM symbiosis (Figure 2). Rhizobium symbiosis leads to increased biosynthesis of cytokinin in shoots, followed by cytokinin transport to the roots, which reduces nodulation events [29]. A recent study shows that this suppression of nodulation requires type-B response regulators, which are essential components of the canonical cytokinin response pathway [30]. Thus, like AMF symbiosis, cytokinin suppresses further symbiotic engagement (i.e., nodulation) when the nutrient uptake is sufficient to favor an increase in the shoot/root growth ratio.
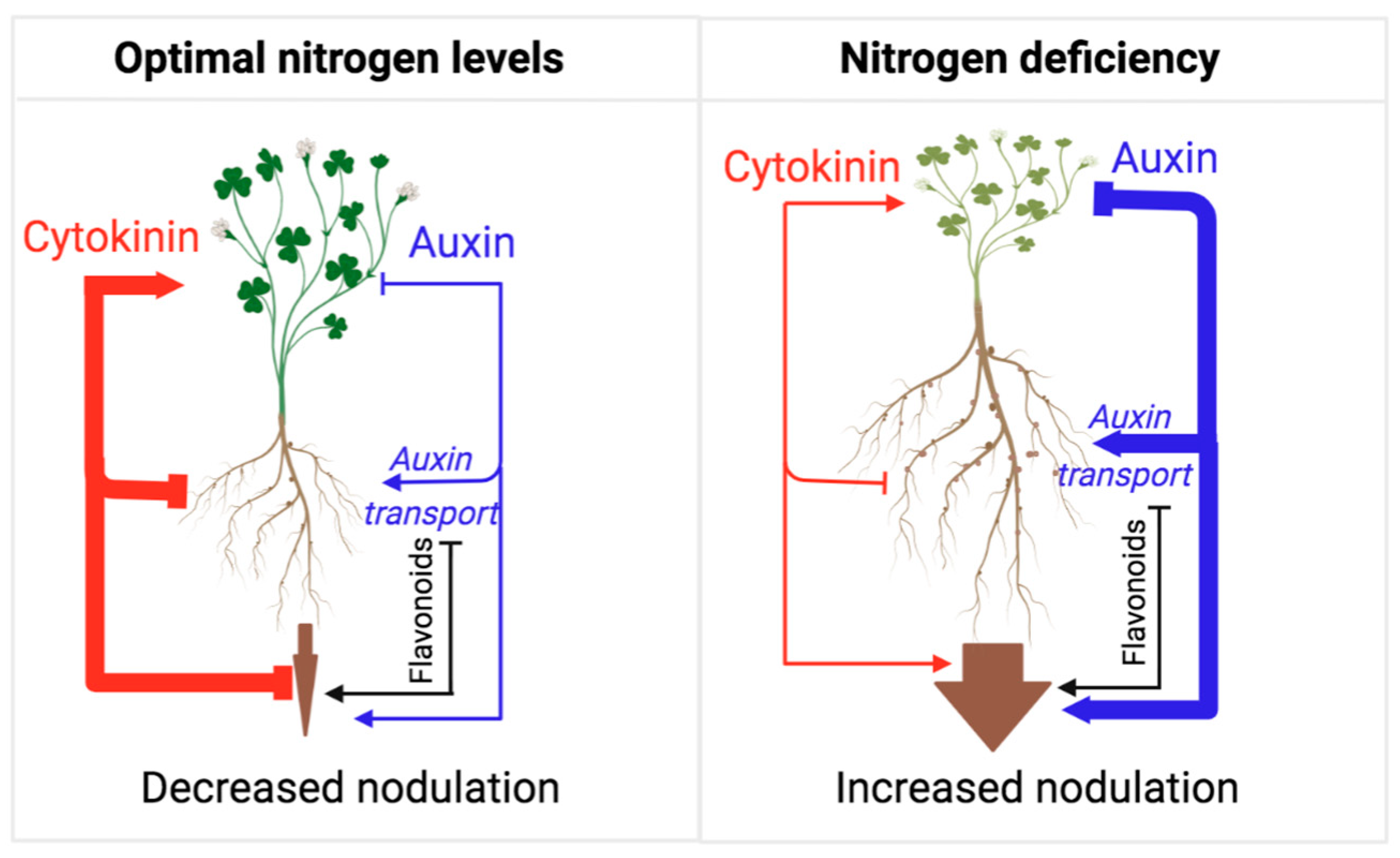
Figure 2. Model representing the actions of auxin and cytokinin on root nodulation from the perspective of adaptive control of the shoot/root growth ratio. As long as the plant perceives nitrogen deficiency in the soil, nodulation frequency is high, mirroring a low shoot/root growth ratio and the allocation of resources towards root growth and nodulation. Under nitrogen-deficient conditions, auxin action is high, which represses cytokinin action and shoot growth in favor of root growth and nodulation. Under nitrogen-sufficient conditions, auxin action is reduced, and cytokinin action is released to promote shoot growth and inhibit root growth and nodulation. This mechanism ensures optimal resource allocation for shoot growth and reproduction under optimal nitrogen conditions. Flavonoids play an essential role in this process, as they promote several stages in this symbiotic interaction and thus help with the auxin-dependent promotion of nitrogen fixation.
This entry is adapted from the peer-reviewed paper 10.3390/plants12030517
References
- Feijen, F.A.A.; Vos, R.A.; Nuytinck, J.; Merckx, V.S.F.T. Evolutionary dynamics of mycorrhizal symbiosis in land plant diversification. Sci. Rep. 2018, 8, 10698.
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Genet. 2008, 6, 763–775.
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16.
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363.
- Fusconi, A. Regulation of root morphogenesis in arbuscular mycorrhizae: What role do fungal exudates, phosphate, sugars and hormones play in lateral root formation? Ann. Bot. 2013, 113, 19–33.
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules 2007, 12, 1290–1306.
- Tian, B.; Pei, Y.; Huang, W.; Ding, J.; Siemann, E. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J. 2021, 15, 1919–1930.
- Nascimento, L.B.D.S.; Tattini, M. Beyond photoprotection: The multifarious roles of flavonoids in plant terrestrialization. Int. J. Mol. Sci. 2022, 23, 5284.
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How auxin and cytokinin phytohormones modulate root microbe interactions. Front. Plant Sci. 2016, 7, 1240.
- Barker, S.J.; Tagu, D. The roles of auxins and cytokinins in mycorrhizal symbioses. J. Plant Growth Regul. 2000, 19, 144–154.
- Kurepa, J.; Smalle, J.A. Auxin/Cytokinin antagonistic control of the shoot/root growth ratio and its relevance for adaptation to drought and nutrient deficiency stresses. Int. J. Mol. Sci. 2022, 23, 1933.
- Pozo, M.J.; Lopez-Raez, J.A.; Azcon, C.; Garrido, J.G. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436.
- Cosme, M.; Ramireddy, E.; Franken, P.; Schmülling, T.; Wurst, S. Shoot- and root-borne cytokinin influences arbuscular mycorrhizal symbiosis. Mycorrhiza 2016, 26, 709–720.
- Ezawa, T.; Saito, K. How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol. 2018, 220, 1116–1121.
- Breuillin, F.; Schramm, J.; Hajirezaei, M.; Ahkami, A.; Favre, P.; Druege, U.; Hause, B.; Bucher, M.; Kretzschmar, T.; Bossolini, E.; et al. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J. 2010, 64, 1002–1017.
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Genet. 2018, 16, 291–303.
- Coba de la Peña, T.; Fedorova, E.; Pueyo, J.J.; Lucas, M.M. The symbiosome: Legume and rhizobia co-evolution toward a nitrogen-fixing organelle? Front. Plant Sci. 2017, 8, 2229.
- Masson-Boivin, C.; Sachs, J.L. Symbiotic nitrogen fixation by rhizobia—The roots of a success story. Curr. Opin. Plant Biol. 2018, 44, 7–15.
- Dong, W.; Song, Y. The Significance of flavonoids in the process of biological nitrogen fixation. Int. J. Mol. Sci. 2020, 21, 5926.
- Oldroyd, G.E.D. Speak, friend and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263.
- Compton, K.K.; Hildreth, S.B.; Helm, R.F.; Scharf, B.E. An updated perspective on Sinorhizobium meliloti chemotaxis to alfalfa flavonoids. Front. Microbiol. 2020, 11, 581482.
- Compton, K.K.; Scharf, B.E. Rhizobial chemoattractants, the taste and preferences of legume symbionts. Front. Plant Sci. 2021, 12, 686465.
- Ghantasala, S.; Choudhury, S.R. Nod factor perception: An integrative view of molecular communication during legume symbiosis. Plant Mol. Biol. 2022, 110, 485–509.
- Liu, C.-W.; Murray, J.D. The Role of flavonoids in nodulation host-range specificity: An update. Plants 2016, 5, 33.
- Wang, Q.; Liu, J.; Zhu, H. Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Front. Plant Sci. 2018, 9, 313.
- Ng, J.L.P.; Hassan, S.; Truong, T.T.; Hocart, C.H.; Laffont, C.; Frugier, F.; Mathesius, U. Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 2015, 27, 2210–2226.
- Plet, J.; Wasson, A.; Ariel, F.; Le Signor, C.; Baker, D.; Mathesius, U.; Crespi, M.; Frugier, F. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 2011, 65, 622–633.
- Vernié, T.; Kim, J.; Frances, L.; Ding, Y.; Sun, J.; Guan, D.; Niebel, A.; Gifford, M.L.; de Carvalho-Niebel, F.; Oldroyd, G.E. The NIN transcription factor coordinates diverse nodulation programs in different tissues of the Medicago truncatula Root. Plant Cell 2015, 27, 3410–3424.
- Sasaki, T.; Suzaki, T.; Soyano, T.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Shoot-derived cytokinins systemically regulate root nodulation. Nat. Commun. 2014, 5, 4983.
- Chen, J.; Wang, Z.; Wang, L.; Hu, Y.; Yan, Q.; Lu, J.; Ren, Z.; Hong, Y.; Ji, H.; Wang, H.; et al. The B-type response regulator GmRR11d mediates systemic inhibition of symbiotic nodulation. Nat. Commun. 2022, 13, 7661.
This entry is offline, you can click here to edit this entry!
