The biological lipid membrane is the key element for the maintenance of cell architecture and physiology. Lipid membranes act as a barrier separating the inner cellular space from the outer environment and further helping in the transmission of signals across the cell boundary. The correct composition and structure of cell membranes define key pathophysiological aspects of cells.
- Membrane
- lipids
- monolayers
- bilayers
- vesicles
- liposomes
1. Introduction
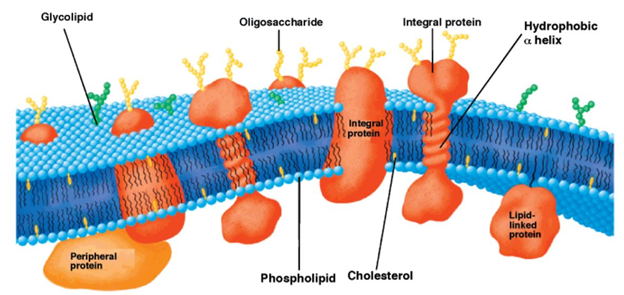
Throughout the biological world, a thick lipid bilayer typically serves as a boundary between life and death for individual cells where the thickness of the membrane is critical in hydrophobic matching and is dependent on lipid properties and is majorly modulated by the extent of cholesterol present in the membrane. A detailed model of the composition and structure of lipid membranes has been developed with the help of biophysical and biochemical findings [1][2]. The fluid mosaic model by Singer and Nicolson describing the structure of the lipid membrane has encouraged other researchers to study membranes and the role of each of their components. The fluid lipid bilayer confers unique physical properties to the cell by interacting with the proteins and thereby creating a variety of domains based on the lipid component [3]. The nature of the association between a certain lipid and protein and the type of lipid that has access to the protein in a certain domain has the potential to modify the activity of the protein [4]. Biomembranes have the basic amphipathic phospholipid bilayer structure wherein the distinct set of proteins (integral and peripheral) is associated with the membrane largely responsible for the unique activities of each type of cellular membrane. Another important class of membrane lipids includes cholesterol being a part of both membrane leaflets helping to modulate the membrane fluidity as seen in Figure 1 [5][6]. The bulk of the membrane’s lipid matrix is constituted by phospholipids which include glycerophospholipid being the major structural lipid followed by the second most abundant structural lipid, sphingolipids [3]. The multifaceted role of lipid membranes can be seen in physiological processes such as cell-cell adhesion, the transport of molecules and ions across membranes, triggering of signal transduction pathways, and cell metabolism.
Figure 1. Schematic representation of the bilayer fluid mosaic model of the cell membrane. Integral proteins are embedded in the bilayer composed of phospholipids and cholesterol. Reproduced with permission from reference [6].
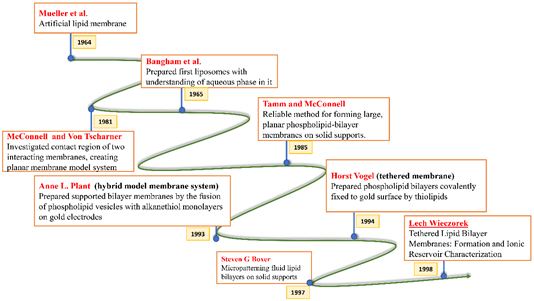
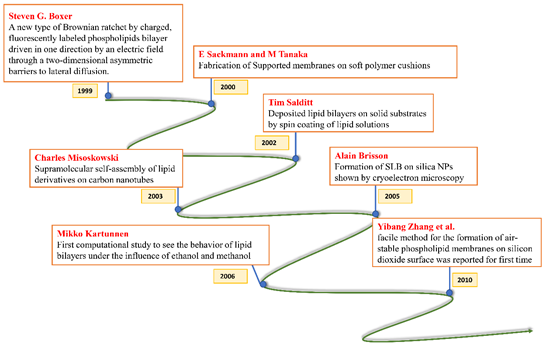
All these crucial roles of biological membranes prompted the development of artificial lipid membrane models to diversify their scope in the field of biosensor development, drug testing, drug discovery, or as simplified model systems for studying the dynamics of the biological membrane [7]. The cell membrane is a research frontier that has been explored for many years and has never failed to intrigue scientists with its prospects for future insight. Model membrane chemistry began with observations of the spreading of oils and fats on the water surface dating back to the eighteenth century. The observations were explored further in 1917 by Irving Langmuir in terms of measuring the surface pressure exerted by the monomolecular layer as the lipid or amphiphilic molecules spread on the water surface. Langmuir further calculated the area per lipid molecule and inferred the flexibility of the hydrocarbon chains [8]. Natural cell membranes are complex structures and pose many problems when studied in their native form. Therefore, simpler lipid model systems are desirable to study cell membrane components and their interaction with biological molecules. Simple cell membrane models span from monolayers at the air-water interface to solid-supported lipid bilayers with ever-growing new formulation techniques involving tethering, hybrid membranes, intact liposomes, use of nanostructures, polymer cushions, and many more ideas [9]. There have been immense contributions in the field of mimicking cell membranes via various unique methods. A timeline showing these developments is illustrated below in Figure 2.
Figure 2. Timeline showing many of the important efforts to mimic the biological cell membrane.
There are several methods for the construction, stabilization, and functionalization of artificial lipid membranes. Each platform has its pros and cons for serving the respective future scope of the intended research. Several synthetic schemes are described below.
2. Different Methods of Formation of Lipid Membrane Models
2.1 Liposomes to Lipid Bilayer - Direct Vesicle Fusion
Biological model membranes have been studied for the past few decades with liposomes and black lipid membranes being the predominant model systems. The method of fusion dates back to the 1980s when membrane-like structures were produced by transferring liposomes to both hydrophilic and hydrophobic surfaces. The sequence of events starting from adsorption to the final bilayer or monolayer formation can be influenced by various parameters such as the surface, the size, and the lipid composition of the liposomes along with various experimental conditions. It is also essential that the membrane proteins acquire the same orientation after the formation of the membrane. The development of the atomic force microscope (AFM) has helped researchers to observe events such as structural changes via enzymatic degradation by sequential imaging in situ under specific conditions [10]. Unilamellar liposomes of diameter 200–300 nm can be prepared from lipid mixtures using the detergent depletion technique. The strategy involves the dissolution of lipids in an organic solvent such as chloroform followed by drying the solvent by flushing nitrogen gas. After complete drying under vacuum, the vessel has to be warmed and preheated in a buffer along with surfactant added to dissolve lipids and form mixed micelles. The dispersion with a specific phospholipid concentration is then recirculated over the support at a constant flow rate for a couple of hours at room temperature [11]. Work has been done to study the structural integrity of unilamellar liposomes upon freezing and thawing. Closely looking into the synthesis as described above, it can be concluded that mechanical agitation is enough to overcome the transition barrier from a suspension of lipid into a suspension of small unilamellar vesicles. However, freezing and thawing are responsible for the partial reversal of this transition. In the study, this loss of integrity was monitored by measuring the change in turbidity, and by loss of energy transfer between fluorescent probes incorporated in the bilayer membrane [12].
2.2. Monolayers at the Air-Water Interface
Lipid films at the air-water interface are used as model systems to[13][14] study the physical properties of membranes and lipid interactions with other important components of biological membranes. Most of the studies using monolayers of fatty acids and lipids at the air-water interface are undertaken using a trough and a rhombus assembly on the stage of a microscope. Surface-pressure is measured using a Wilhelmy plate attached to a torsion balance . Insoluble monolayers can be investigated using the Langmuir trough, which is used to generate pressure-area (Π-A) isotherms whose kink and plateau analysis provides an easy route to learn about the phase and ordering transitions experienced by the monolayer. The procedure to obtain an isotherm of the given monolayer is to spread the film, evaporate the solvent and then compress the film using a moving barrier at modest speeds [15].
Current biomimetic models postulate non-random lipid mixing in each leaflet of the bilayer giving rise to microdomains with special physical and functional properties. Analysis of interfacial potential and surface pressure as a function of the cross-sectional molecular area can give a clear picture of the hydrocarbon chain ordering, lateral compressibility, and dipole effects of the lipid layer upon biological interactions [16]. Using this approach, the condensing effect of cholesterol on the liquid-expanded phase of different molecular species of galactosylceramide (GalCer) with homogeneous acyl chains was studied. Incorporation of cholesterol reduced the trans-gauche isomerization about the C–C bonds in liquid-disordered acyl chains of GalCer resulting in significant condensation due to gain in van der Waals forces between the molecules [17].
2.3. Langmuir–Blodgett Type Approaches
The Langmuir–Blodgett (LB) transfer technique is a well-established method that dates to 1917, pioneered by Irving Langmuir and Katherine Blodgett to prepare supported monolayers, bilayers, and multilayers. The LB method is widely used for creating supported monolayers and bilayers to serve as model membrane systems of controlled composition and physical state, given that the precursor monolayers can be spread on the water surface with precisely controlled composition and can be transferred to a substrate at a set surface pressure corresponding to a desired physical state. The monolayer of lipid molecules at the air-water interface is to be compressed using a moving barrier to control the lipid phase. Lipid films are then transferred onto solid substrata using either LB or Schaefer transfer method [18]. Katherine Blodgett expanded the method to multilayers with repeated dipping of the substrate through the air-water interface [19]. Phospholipid bilayers have been formed on various substrates such as glass, quartz, and silicon surfaces by transferring monolayers at a pressure of approximately 40 dyne cm−1 from the air-water interface to the solid substrate [20]. It is very important to transfer a monolayer formed at the air-water interface onto a solid substrate for advancement in the study of monolayers and thin films. Microscopic and spectroscopic tools are adapted and used for characterizing the structural It was reported that structural changes in lipid monolayers occur during LB transfer due to monolayer/substrate interactions. During the slow transfer, the domain-free gap was observed due to substrate mediated condensation of the monolayer in the moment of its deposition [21].
2.4. Spin-Coated Lipid Bilayers and Their Characterization
Interaction between membranes and biological molecules with an emphasis on biochemical and biophysical aspects under well-controlled conditions can be very well understood with the knowledge of solid-supported lipid bilayers. Several routes have been explored for the preparation of solid-supported bilayers such as vesicle fusion, Langmuir–Blodgett, and Langmuir–Schaeffer, and spreading from organic solutions. Studies have shown the importance of thermal stability and orientational alignment of the layers on the solid support. Spin coating is a valuable technique for preparing stacks of small, uniform, and well-oriented bilayers on support [22]. This method is useful for preparing multiple bilayers oriented on a solid substrate. With access to a spin coater, the creation of the thin film of stacked bilayers is a fairly straightforward procedure. In this technique, a drop of solution for making the coating is added onto the substrate and the substrate was rotated until the solvent evaporated to produce a uniform biomimetic layer suitable for investigations with X-ray reflectivity. It was found that the number of bilayers varied linearly with change in lipid concentration and upon hydration, the stability deteriorated [23]. An alternative to obtaining dry, air-stable, and high-quality lipid multilayers is the use of spin-coating techniques. This low-dimensionality system is defect-free and gives rise to morphologically stable lipid layers in dry air conditions [24].
2.5. Vesicle Fusion Method Leading to Supported Lipid Bilayers (SLBs)
Vesicle fusion is an easy and reliable method to form supported lipid bilayers from zwitterionic vesicles on siliceous substrates. High-quality SLBs are produced via a vesicle fusion method that has three stages namely, vesicle adsorption on the substrate followed by vesicle rupture, fusion, and bilayer spreading. Complex SLBs can be created by optimizing the experimental conditions, temperature, buffer selection, and the use of α-helical peptides which enhances the rate of vesicle fusion [25]. A key parameter for SLB formation via vesicle fusion is the deposition temperature as few lipids exist in the gel phase at room temperature. Moreover, the outcome of vesicle fusion depends on the solution pH, ionic strength, presence of divalent cations, nature of lipids, and the substrate over which the fusion takes place [26]. Successful vesicle fusion depends on high-quality vesicle preparation, and the method works with a narrow range of supports and lipid composition. Industrially useful surfaces such as gold and titanium oxide vesicles typically adsorb but tend to remain intact [27]. Studies have been done to see the effect of the geometric structure of lipids on vesicle fusion. Intrinsic curvature of a vesicle’s component lipids is the deciding parameter in the events that occur after the vesicle interacts with the substrate. It was seen that lipid shape will determine the stability and feasibility of the transient substructures that further leads to SLB formation [28]. Unilamellar vesicle preparation along with quality control are two essential requirements that involve technical skill and resources and are needed to achieve a planar lipid bilayer via vesicle fusion. Furthermore, vesicle rupture occurs on a limited set of substrates and this necessitates the need for an alternative method. Recently, the process of solvent-assisted lipid bilayer (SALB) formation on silicon dioxide and gold has been explored [29].
3. Conclusions
We began our discussion by throwing some light on liposome fusion, monolayers at the air-water interface, LB-LS techniques, and other methods involving self-assembly. Over the past few years, biomimetic models of lipid membranes have been extensively worked upon involving tethered and hybrid systems and various techniques such as AFM, Quartz crystal microbalance (QCM), and fluorescence spectroscopy have been used to fully characterize the formation process. The effect of various parameters such as type of substrate selected for the formation of SLB, lipid concentration in the organic solvent for vesicle formation, temperature, and the methodology employed have been seen to have had a pronounced effect on the membrane stability and structural features. It appears that biomimetic membranes have a huge potential for biomedical applications. We feel that it is extremely timely to have a deeper understanding of the structure and functions of biological membranes to create a biomimetic system that can be used to design biosensors and drug carriers.
This entry is adapted from the peer-reviewed paper 10.3390/coatings10100981
References
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: where they are and how they behave. Nature reviews. Molecular cell biology 2008, 9, 112-124.
- Mitra, K.; Ubarretxena-Belandia, I.; Taguchi, T.; Warren, G.; Engelman, D.M. Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proceedings of the National Academy of Sciences 2004, 101, 4083-4088.
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int J Mol Sci 2019, 20, 2167.
- Boesze-Battaglia, K.; Schimmel, R. Cell membrane lipid composition and distribution: implications for cell function and lessons learned from photoreceptors and platelets. Journal of experimental biology 1997, 200, 2927-2936.
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Biomembranes: Structural organization and basic functions. In Molecular Cell Biology. 4th edition, WH Freeman: 2000.
- Percec, V.; Bera, T.K. Cell Membrane as a Model for the Design of Ion-Active Nanostructured Supramolecular Systems. Biomacromolecules 2002, 3, 167-181.
- Siontorou, C.G.; Nikoleli, G.-P.; Nikolelis, D.P.; Karapetis, S.K. Artificial lipid membranes: past, present, and future. Membranes 2017, 7, 38.
- Edidin, M. Lipids on the frontier: a century of cell-membrane bilayers. Nature Reviews Molecular Cell Biology 2003, 4, 414-418.
- Lind, T.K.; Cárdenas, M. Understanding the formation of supported lipid bilayers via vesicle fusion—A case that exemplifies the need for the complementary method approach. Biointerphases 2016, 11, 020801.
- Jass, J.; Tjärnhage, T.; Puu, G. From liposomes to supported, planar bilayer structures on hydrophilic and hydrophobic surfaces: an atomic force microscopy study. Biophysical journal 2000, 79, 3153-3163.
- Puu, G.; Gustafson, I. Planar lipid bilayers on solid supports from liposomes–factors of importance for kinetics and stability. Biochimica et Biophysica Acta (BBA)-Biomembranes 1997, 1327, 149-161.
- Strauss, G.; Ingenito, E. Stabilization of liposome bilayers to freezing and thawing: Effects of cryoprotective agents and membrane proteins. Cryobiology 1980, 17, 508-515.
- McConnell, H.M.; Tamm, L.K.; Weis, R.M. Periodic structures in lipid monolayer phase transitions. Proceedings of the National Academy of Sciences 1984, 81, 3249-3253.
- von Tscharner, V.; McConnell, H.M. An alternative view of phospholipid phase behavior at the air-water interface. Microscope and film balance studies. Biophysical journal 1981, 36, 409-419.
- Klopfer, K.; Vanderlick, T. Isotherms of dipalmitoylphosphatidylcholine (DPPC) monolayers: features revealed and features obscured. Journal of colloid and interface science 1996, 182, 220-229.
- Brown, R.E.; Brockman, H.L. Using monomolecular films to characterize lipid lateral interactions. In Lipid Rafts, Springer: 2007; pp. 41-58.
- Ali, S.; Smaby, J.M.; Brockman, H.L.; Brown, R.E. Cholesterol's interfacial interactions with galactosylceramides. Biochemistry 1994, 33, 2900-2906.
- Dufrêne, Y.F.; Lee, G.U. Advances in the characterization of supported lipid films with the atomic force microscope. Biochimica et Biophysica Acta (BBA)-Biomembranes 2000, 1509, 14-41.
- Kurniawan, J.; Ventrici de Souza, J.o.F.; Dang, A.T.; Liu, G.-y.; Kuhl, T.L. Preparation and Characterization of Solid-Supported Lipid Bilayers Formed by Langmuir–Blodgett Deposition: A Tutorial. Langmuir 2018, 34, 15622-15639.
- Tamm, L.K.; McConnell, H.M. Supported phospholipid bilayers. Biophysical journal 1985, 47, 105.
- Riegler, H.; Spratte, K. Structural changes in lipid monolayers during the Langmuir-Blodgett transfer due to substrate/monolayer interactions. Thin Solid Films 1992, 210, 9-12.
- Mennicke, U.; Salditt, T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir 2002, 18, 8172-8177.
- Simonsen, A.C.; Bagatolli, L.A. Structure of spin-coated lipid films and domain formation in supported membranes formed by hydration. Langmuir 2004, 20, 9720-9728.
- Dols-Perez, A.; Fumagalli, L.; Simonsen, A.C.; Gomila, G. Ultrathin Spin-Coated Dioleoylphosphatidylcholine Lipid Layers in Dry Conditions: A Combined Atomic Force Microscopy and Nanomechanical Study. Langmuir 2011, 27, 13165-13172.
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model cell membranes: Techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Current opinion in colloid & interface science 2013, 18, 448-458.
- Lind, T.K.; Cárdenas, M.; Wacklin, H.P. Formation of supported lipid bilayers by vesicle fusion: effect of deposition temperature. Langmuir 2014, 30, 7259-7263.
- Jackman, J.A.; Cho, N.-J. Supported Lipid Bilayer Formation: Beyond Vesicle Fusion. Langmuir 2020, 36, 1387-1400.
- Hamai, C.; Yang, T.; Kataoka, S.; Cremer, P.S.; Musser, S.M. Effect of average phospholipid curvature on supported bilayer formation on glass by vesicle fusion. Biophysical journal 2006, 90, 1241-1248.
- Tabaei, S.R.; Choi, J.-H.; Haw Zan, G.; Zhdanov, V.P.; Cho, N.-J. Solvent-assisted lipid bilayer formation on silicon dioxide and gold. Langmuir 2014, 30, 10363-10373.