Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Choroidal neovascularizations are historically associated with exudative macular degeneration, nonetheless, they have been observed in nevus, melanoma, osteoma, and hemangioma involving the choroid and retina.
- choroidal neovascularization
- tumor
- choroid
1. Choroidal Neovascularization Mechanism in In-Vivo and In-Vitro Models
Choroidal neovascularization (CNV) is historically associated with exudative macular degeneration, which is characterized by the deposition of insoluble material in the retinal layers, choriocapillaris thinning, and alteration in Bruch’s membrane thickness. There is an accumulation of lipoproteins, which leads to retinal pigment epithelium (RPE) and photoreceptor atrophy, para-inflammation, and hypoxia. Together, they eventually lead to the secretion of vascular endothelial growth factor (VEGF) from RPE, photoreceptors, and immune cells, which promotes neovascularization [1][2][3]. Exudative age-related macular degeneration (AMD) is a consequence secondary to CNV [4] as shown in Figure 1.
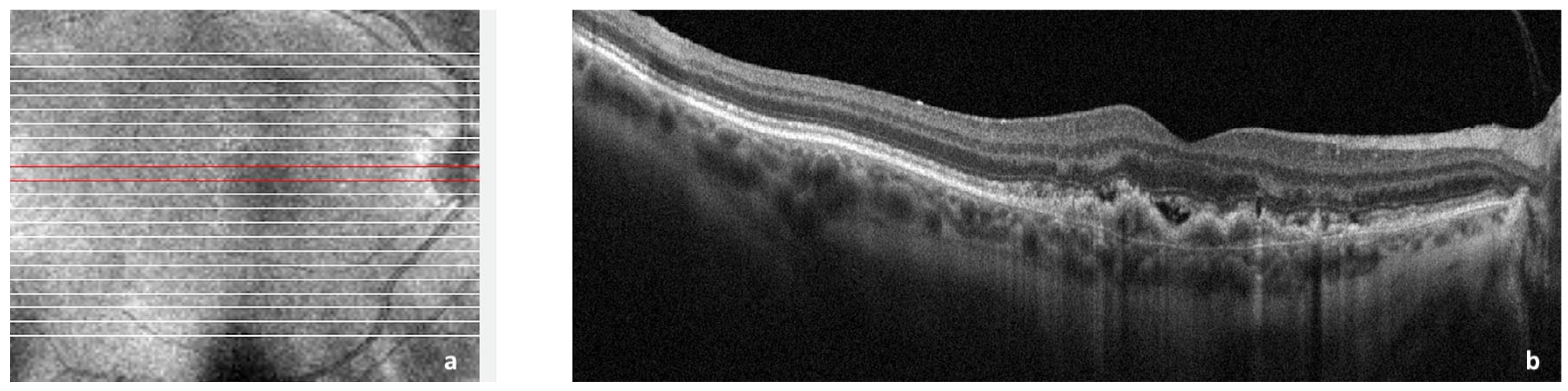
Figure 1. Spectral domain optical coherence tomography (SDOCT) of the macular area showing choroidal neovascularization (CNV) secondary to age-related macular degeneration. (a): Image of the macula showing overlying raster scan; (b): cross-sectional SDOCT scan showing retinal pigment epithelium (RPE) elevation due to CNV underlying the RPE and adjacent subretinal fluid.
The first classification of the different types and growth patterns was built in 1991 around fluorescein angiography (FA) leakage evidence dividing the neovascular membranes in occult or classic [5]. The occult neovascular membrane is an ingrowth of a neovascular complex initially from the choriocapillaris, into and within the sub-RPE space, that shows a stippled hyperfluorescence, which expands and becomes more evident only in the later phases of FA; classic neovascular membrane originates from the choroid and traverses Bruch’s membrane and the RPE; hence, it may be detected in the very early phase of FA [4][5]. In the following years, the histologic subtypes were classified as CNV type 1, where the CNV is located below the RPE, and CNV type 2, where the CNV is located above the RPE to describe occult and classic neovascularization, respectively [4]. Both are mainly composed of fibrovascular tissue. A combined subtype of type 1 and 2 was also identified [6][7].
2. Vascular Mechanisms in Choroidal Neovascularization
The first and leading hypothesis in 1987 was that all new vessels in CNV arise from pre-existing choroidal vasculature [8]. In the 1990s, bone marrow circulating progenitor cells were identified as contributors to adult vasculogenesis [9][10]. With the laser photocoagulation-induced injury to the choroid described above, researchers transplanted enhanced green fluorescent protein (EGFP)-expressing bone marrow cells from EGFP donor mice into laser-treated mice. Green fluorescent protein recruited cells (GFP+) were quantified in the choroidal vasculatures or in Bruch’s membrane injury sites, showing different levels of contribution in CNV [11][12]. The proportion of GFP+ cells contributing to lesion endothelial cells was observed to be dependent on the stage of CNV [13][14], and mobilized adult hematopoietic stem cells were observed to be able to form endothelial cells subsequently incorporated into choroidal neovasculature [15]. Similar hemangioblast activity was also observed in the murine model [16] and in humans. In the latter, the presence of bone marrow-derived progenitor was identified in excised CNV sections by tracing the AC133 marker of hematopoietic stem cells and bone marrow-derived progenitors [17].
3. Molecular Mechanisms
VEGF is found in several isoforms, VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206, and is a strong angiogenic molecule capable of stimulating proliferation, migration, and enhancing vascular permeability of endothelial cells [18][19]. Dysregulation of VEGF is known as one of the main steps in pathological angiogenesis [20]. In physiological conditions, VEGF and FGF2 are released by the RPE during fetal development to promote the development of the choriocapillaris [21]. Furthermore, VEGF is required for the formation of fenestrations in the choriocapillaris [22] in order to allow macromolecule transit in and out of choroidal circulation [21]. Under normal conditions, VEGF is kept at a basal level, but in pathological reactive conditions, such as CNV, VEGF levels are significantly increased [20]. The VEGF isoform that is predominantly involved in pathological angiogenesis is VEGF164/165 [23]. In addition to VEGFs’ stimulating role in angiogenesis, their elevated level in the RPE leads to barrier disruption, which could further increase neovascularization chances [22] as illustrated in Figure 2.
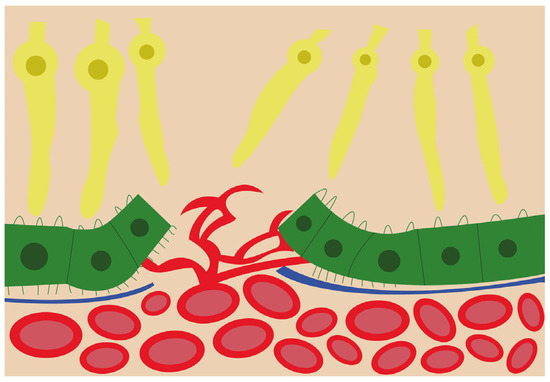
Figure 2. Schematic view of the choroidal neovascularization process. The new blood vessels from the choroid have breached the retinal pigment epithelium and are branching and invading the retina.
4. CNV Associated with Choroidal Nevi and Melanomas
Choroidal nevi are benign pigmented tumors that can only occasionally cause loss of visual acuity [24]. Over time, these lesions may induce secondary changes in the pigment epithelium and lead to the formation of drusen, serous retinal detachment, and proliferation of CNV [25]. In 2004, Zografos et al. [26] described 22 cases of choroidal nevi inducing the formation of a neovascular membrane. The CNV was situated close to the center of the pigmented nevus, was not larger than the nevus in 20 cases, and extended beyond the edge of the lesion in the other 2 cases. CNV was classic in all cases.
Choroidal melanomas are the most common primary intraocular malignancy in adults [27]. The Collaborative Ocular Melanoma Study (COMS) found that choroidal melanomas are more common in Caucasians and that the mean age at diagnosis is 60 years [28]. These melanomas are usually located posterior to the ciliary body. Typically asymptomatic, they are most commonly found during routine ophthalmic examination. When symptomatic, depending on the tumor location, they can induce “flashing lights” due to tumor-induced exudative retinal detachment or metamorphopsia due to subfoveal tumor [29].
Accumulations of lipofuscin and melanolipofuscin as yellow/orange pigment can be visualized on the surface of the melanoma. An exudative subretinal fluid can also be also observed overlying the primary tumor, indicating incontinent tumor blood vessels leaking beneath the retina [29]. Lubin et al. in 1982 [30], described a case of malignant choroidal melanoma associated with CNV, becoming the first histologic verification to appear in the literature. Guerin et al. in 2006 [31], examined a series of choroidal melanoma to study the frequency and particular histological tumor characteristics in melanoma-associated CNV. Microscopic evidence of CNVs was found in 14 of the 229 globes examined. The overall incidence was therefore 6%. Each case was examined after multiple sections from different planes were taken and stained with hematoxylin and eosin (H&E), diastase-periodic acid Schiff (DPAS), and Gomori Trichrome (GOM). Three CNVs were located over the tumor apex, two at both sides of the apex, six at the side of the tumor, and the last three over the tumor edge. In 2013, an unusual case of CNV complicating a choroidal nevus in a 16-year-old patient was also reported [32].
5. CNV in Choroidal Osteoma
Choroidal osteoma is a benign intraocular tumor made of mature bone that replaces the entire choroid. This tumor typically appears as a yellow–orange plaque on retinal examination. Usually found in the juxta papillary or macular region [33], it manifests as a unilateral lesion in young females. The etiology and pathogenesis of choroidal osteoma are poorly understood, but after many years and several studies, it was eventually recognized to be related to CNV [34][35]. In 1998, Aylward et al. [36] observed the long-term outcome of 36 patients with choroidal osteoma, showing a moderate risk for the development of CNV. Several years later, in 2005, Shields et al. [37] conducted an extensive retrospective nonrandomized study evaluating choroidal osteoma for tumor growth, tumor decalcification, and CNV. CNV was associated with a choroidal osteoma in 21% of eyes on 1-year follow-up, but in 46% of eyes on 20-year follow-up. The greatest risk for the development of CNV was an irregular surface and an overlying hemorrhage. Other case reports corroborated the correlation between choroidal osteoma and the development of CNV after several years [38] or even in the case of bilateral osteomas [39]. CNV complicating a choroidal osteoma is typically “classic”, as previously described, but Kim et al. in 2020 [40], described a case of polypoidal choroidal vasculopathy (PCV), which is characterized by more complex and aggressive branching choroidal vessels with terminal (polyp-like) aneurysmal dilations. The authors observed irregular RPE elevations over the regions of decalcification around the optic nerve, suggesting that a quiescent CNV can progress to PCV.
6. CNV in Choroidal Hemangioma
CNV is a rare event in association with circumscribed choroidal hemangioma (CCH) or after its treatment. Ruby et al. in 1992 [41], reported two patients with choroidal hemangiomas developing CNV; one patient had Sturge–Weber syndrome (SWS) with a unilateral diffuse choroidal hemangioma (DCH). Shields et al. in 2001 [42], observed that only three CCH patients had concomitant CNV in 200 cases examined. An even rarer event was observed in a CCH with HIV infection by Hua et al. in 2014 [43]. Given the case, overall complexity, and the wide range of cofactors involved the authors suggested that various mechanisms may be responsible for the CNV formation. In particular, the authors considered that a continuous vascular leakage of CCH may facilitate angiogenesis led by plasma proteins and fibrin. Furthermore, CCH could stimulate the release of angiogenic factors, due to the constant low-grade inflammation and ischemia. Another possibility is that CNV can also be stimulated by laser-induced necrosis of the tumor and VEGF released after photo-dynamic treatment (PDT). Several complications including RPE alterations and photoreceptor loss have been described after PDT therapy. Despite some evidence showing the development of retinal neovascularization [44] and PCV [45] after PDT, its role in CCH-related CNV is still unclear.
A peculiar case is represented by the SWS, a neuro-oculo-cutaneous hemangiomatosis where DCH is a key finding [46]. In SWS, the eye is involved in more than 50% of patients and the main features are glaucoma and DCH with several possible complications [47]. SWS genetic studies showed that DCH occurs sporadically from an activating mutation in GNAQ at codon R183 [48]. Mutations in GNAQ or GNA11 result in the upregulation of the mitogen-activated protein kinase, which in turn results in cellular proliferation [49]. In 2019, Bichsel et al. [50] showed that the mutation found in most sporadic capillary malformations, GNAQ R183Q, was present in the choroidal vessels at a similar frequency to that found in SWS brain tissue, suggesting an analogous choroidal capillary malformation cause. Anti-VEGF use alone is neither resolutive nor indicated, but recent publications are showing the utility of adding anti-VEGF agents to PDT to counter the effect of PDT-induced high VEGF levels [51]. These results indicate that CNV and choroidal capillary malformations follow different pathways, but the development of new vessel complexes in SWS could still represent a potential complication [41].
7. CNV in Primary Vitreous Retinal Lymphoma
Primary vitreous retinal lymphoma (PVRL) is a rare intraocular malignancy and CNV has not been reported regularly as a complication. Ma et al. in 2020 [52], described a case of PVRL characterized by subretinal hyperreflective material and complicated with CNV. Of note, the authors admitted that the CNV could have existed since the first visit but was not recognized on instrumental imaging due to RPE perturbations. Furthermore, these authors hypothesized that the growth of CNV could be related to RPE hypoxia. Lymphoma cells infiltrate and proliferate under the retina producing elevated levels of IL-10 [53]. Il-10 serves as a growth factor of B-cells and the resulting massive cell aggregates under the RPE may affect oxygen diffusion. The natural response would be an upregulation of VEGF. Unfortunately, the evidence in the literature is so scarce that it is impossible to determine a definitive correlation between PVRL and CNV at the moment.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24021064
References
- Ng, E.W.M.; Adamis, A.P. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can. J. Ophthalmol. 2005, 40, 352–368.
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primer. 2021, 7, 31.
- Rozing, M.P.; Durhuus, J.A.; Krogh Nielsen, M.; Subhi, Y.; Kirkwood, T.B.; Westendorp, R.G.; Sørensen, T.L. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 2020, 76, 100825.
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636.
- Macular Photocoagulation Study Group. Laser Photocoagulation of Subfoveal Recurrent Neovascular Lesions in Age-Related Macular Degeneration: Results of a Randomized Clinical Trial. Arch. Ophthalmol. 1991, 109, 1232–1241.
- Gass, J.D.M. Biomicroscopic and Histopathologic Considerations Regarding the Feasibility of Surgical Excision of Subfoveal Neovascular Membranes. Am. J. Ophthalmol. 1994, 118, 285–298.
- Lafaut, B.A.; Bartz-Schmidt, K.U.; Broecke, C.V.; Aisenbrey, S.; Laey, J.J.D.; Heimann, K. Clinicopathological correlation in exudative age related macular degeneration: Histological differentiation between classic and occult choroidal neovascularisation. Br. J. Ophthalmol. 2000, 84, 239–243.
- Ishibashi, T.; Miller, H.; Orr, G.; Sorgente, N.; Ryan, S.J. Morphologic observations on experimental subretinal neovascularization in the monkey. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1116–1130.
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967.
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228.
- Sengupta, N.; Caballero, S.; Mames, R.N.; Butler, J.M.; Scott, E.W.; Grant, M.B. The role of adult bone marrow-derived stem cells in choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4908–4913.
- Tomita, M.; Yamada, H.; Adachi, Y.; Cui, Y.; Yamada, E.; Higuchi, A.; Minamino, K.; Suzuki, Y.; Matsumura, M.; Ikehara, S. Choroidal neovascularization is provided by bone marrow cells. Stem Cells Dayt. Ohio 2004, 22, 21–26.
- Espinosa-Heidmann, D.G.; Reinoso, M.A.; Pina, Y.; Csaky, K.G.; Caicedo, A.; Cousins, S.W. Quantitative enumeration of vascular smooth muscle cells and endothelial cells derived from bone marrow precursors in experimental choroidal neovascularization. Exp. Eye Res. 2005, 80, 369–378.
- Hou, H.-Y.; Wang, Y.-S.; Xu, J.-F.; Wang, Y.-C.; Liu, J.-P. The dynamic conduct of bone marrow-derived cells in the choroidal neovascularization microenvironment. Curr. Eye Res. 2006, 31, 1051–1061.
- Chan-Ling, T.; Baxter, L.; Afzal, A.; Sengupta, N.; Caballero, S.; Rosinova, E.; Grant, M.B. Hematopoietic stem cells provide repair functions after laser-induced Bruch’s membrane rupture model of choroidal neovascularization. Am. J. Pathol. 2006, 168, 1031–1044.
- Grant, M.B.; May, W.S.; Caballero, S.; Brown, G.A.J.; Guthrie, S.M.; Mames, R.N.; Byrne, B.J.; Vaught, T.; Spoerri, P.E.; Peck, A.B.; et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat. Med. 2002, 8, 607–612.
- Sheridan, C.M.; Rice, D.; Hiscott, P.S.; Wong, D.; Kent, D.L. The presence of AC133-positive cells suggests a possible role of endothelial progenitor cells in the formation of choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1642–1645.
- Ferrara, N.; Houck, K.A.; Jakeman, L.B.; Winer, J.; Leung, D.W. The vascular endothelial growth factor family of polypeptides. J. Cell. Biochem. 1991, 47, 211–218.
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wie-gand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185.
- Kinnunen, K.; Ylä-Herttuala, S. Vascular endothelial growth factors in retinal and choroidal neovascular diseases. Ann. Med. 2012, 44, 1–17.
- Anand-Apte, B.; Hollyfield, J.G. Developmental Anatomy of the Retinal and Choroidal Vasculature. In Encyclopedia of the Eye; Dartt, D.A., Ed.; Academic Press: Oxford, UK, 2010; pp. 9–15. Available online: https://www.sciencedirect.com/science/article/pii/B978012374203200169X (accessed on 2 January 2023).
- Marneros, A.G.; Fan, J.; Yokoyama, Y.; Gerber, H.P.; Ferrara, N.; Crouch, R.K.; Olsen, B.R. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am. J. Pathol. 2005, 167, 1451–1459.
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Amano, S.; Ogura, Y.; Hida, T.; Oguchi, Y.; Ambati, J.; Miller, J.W.; et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J. Exp. Med. 2003, 198, 483–489.
- Gonder, J.R.; Augsburger, J.J.; McCarthy, E.F.; Shields, J.A. Visual loss associated with choroidal nevi. Ophthalmology 1982, 89, 961–965.
- Rouic, L.L.-L. Mélanome Choroïdien: Aspect Clinique au Diagnostic et après Traitement (Choroidal Melanoma: Clinical Aspect at Diagnosis and after Treatment). Images en Ophtalmologie . Edimark.fr, Vol. I, n. 1. Octo-Bre-Novembre-Décembre 2007. Available online: https://www.edimark.fr/images-ophtalmologie/melanome-choroidien-aspect-clinique-diagnostic-apres-traitement (accessed on 2 January 2023).
- Zografos, L.; Mantel, I.; Schalenbourg, A. Subretinal Choroidal Neovascularization Associated with Choroidal Nevus. Eur. J. Ophthalmol. 2004, 14, 123–131.
- Singh, A.D.; Bergman, L.; Seregard, S. Uveal melanoma: Epidemiologic aspects. Ophthalmol. Clin. N. Am. 2005, 18, 75–84.
- Hu, D.-N.; Yu, G.-P.; McCormick, S.A. Population-based incidence of vulvar and vaginal melanoma in various races and ethnic groups with comparisons to other site-specific melanomas. Melanoma Res. 2010, 20, 153–158.
- Finger, P.T. Eye: Choroidal melanoma, retinoblastoma, ocular adnexal lymphoma and eyelid cancers. In UICC Manual of Clinical Oncology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 726–744. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119013143.ch56 (accessed on 2 January 2023).
- Lubin, J.R.; Gragoudas, E.S.; Albert, D.M. Choroidal Neovascularization Associated with Malignant Melanoma. Acta Ophthalmol. 1982, 60, 412–418.
- Guerin, E.; Hiscott, P.; Damato, B. Choroidal neovascular membrane in a series of cases of malignant melanoma of the choroid. ACTA Ophthalmol. Scand. 2006, 84, 323–327.
- Tuncer, S.; Tugal-Tutkun, I. Choroidal neovascularization secondary to choroidal nevus simulating an inflammatory lesion. Indian J. Ophthalmol. 2013, 61, 305–306.
- Shields, C.L.; Shields, J.A.; Augsburger, J.J. Choroidal osteoma. Surv. Ophthalmol. 1988, 33, 17–27.
- Grand, M.G.; Burgess, D.B.; Singerman, L.J.; Ramsey, J. Choroidal osteoma. Treatment of associated subretinal neovascular membranes. Retina 1984, 4, 84–89.
- Foster, B.S.; Fernandez-Suntay, J.P.; Dryja, T.P.; Jakobiec, F.A.; D’Amico, D.J. Clinicopathologic reports, case reports, and small case series: Surgical removal and histopathologic findings of a subfoveal neovascular membrane associated with choroidal osteoma. Arch. Ophthalmol. 2003, 121, 273–276.
- Aylward, G.W.; Chang, T.S.; Pautler, S.E.; Gass, J.D. A long-term follow-up of choroidal osteoma. Arch. Ophthalmol. 1998, 116, 1337–1341.
- Shields, C.L.; Sun, H.; Demirci, H.; Shields, J.A. Factors predictive of tumor growth, tumor decalcification, choroidal neovascularization, and visual outcome in 74 eyes with choroidal osteoma. Arch. Ophthalmol. 2005, 123, 1658–1666.
- Zhang, Y. Secondary choroidal neovascularization due to choroidal osteoma after 9 years follow-up. BMC Ophthalmol. 2021, 21, 242.
- Naik, A.U.; Raman, R. Bilateral Choroidal Osteomas with Choroidal Neovascularization. JAMA Ophthalmol. 2020, 138, e190059.
- Kim, D.; Ryu, G.; Sagong, M. Polypoidal choroidal vasculopathy as a complication of choroidal osteoma: A case report. Medicine 2020, 99, e19927.
- Ruby, A.J.; Jampol, L.M.; Goldberg, M.F.; Schroeder, R.; Anderson-Nelson, S. Choroidal neovascularization associated with choroidal hemangiomas. Arch. Ophthalmol. 1992, 110, 658–661.
- Shields, C.L.; Honavar, S.G.; Shields, J.A.; Cater, J.; Demirci, H. Circumscribed choroidal hemangioma: Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology 2001, 108, 2237–2248.
- Hua, R.; Zhao, N.; Hu, Y.; Zhang, C.M.; Chen, L. Circumscribed choroidal hemangioma associated with choroidal neovascularization in a HIV-infected case: Photodynamic therapy and intravitreous ranibizumab. Photodiagnosis Photodyn. Ther. 2014, 11, 441–443.
- Leys, A.M.; Silva, R.; Inhoffen, W.; Tatar, O. Neovascular growth following photodynamic therapy for choroidal hemangioma and neovascular regression after intravitreous injection of triamcinolone. Retina 2006, 26, 693–697.
- Tuncer, S.; Demirci, H.; Shields, C.L.; Shields, J.A. Polypoidal choroidal vasculopathy following photodynamic therapy for choroidal hemangioma. Eur. J. Ophthalmol. 2009, 19, 159–162.
- Witschel, H.; Font, R.L. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv. Ophthalmol. 1976, 20, 415–431.
- Singh, A.D.; Kaiser, P.K.; Sears, J.E. Choroidal hemangioma. Ophthalmol. Clin. N. Am. 2005, 18, 151–161.
- Shirley, M.D.; Tang, H.; Gallione, C.J.; Baugher, J.D.; Frelin, L.P.; Cohen, B.; North, P.E.; Marchuk, D.A.; Comi, A.M.; Pevsner, J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 2013, 368, 1971–1979.
- Francis, J.H.; Milman, T.; Grossniklaus, H.; Albert, D.; Folberg, R.; Levitin, G.; Coupland, S.; Catalanotti, F.; Rabady, D.; Kandoth, C.; et al. GNAQ Mutations in Diffuse and Solitary Choroidal Hemangiomas. Ophthalmology 2019, 126, 759–763.
- Bichsel, C.A.; Goss, J.; Alomari, M.; Alexandrescu, S.; Robb, R.; Smith, L.E.; Hochman, M.; Greene, A.K.; Bischoff, J. Association of Somatic GNAQ Mutation with Capillary Malformations in a Case of Choroidal Hemangioma. JAMA Ophthalmol. 2019, 137, 91–95.
- Formisano, M.; di Pippo, M.C.; Scuderi, L.; Abdolrahimzadeh, S. Current concepts on diffuse choroidal hemangioma in Sturge Weber syndrome. Ophthalmic Genet. 2021, 42, 375–382.
- Ma, Y.; Zhao, H.; Peng, X. Choroidal neovascularization as a complication of primary vitreous retinal lymphoma. Eur. J. Ophthalmol. 2021, 31, NP31–NP35.
- Chan, C.-C.; Rubenstein, J.L.; Coupland, S.E.; Davis, J.L.; Harbour, J.W.; Johnston, P.B.; Cassoux, N.; Touitou, V.; Smith, J.R.; Batchelor, T.T.; et al. Primary vitreoretinal lymphoma: A report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncol. 2011, 16, 1589–1599.
This entry is offline, you can click here to edit this entry!
