3. Molecular Mechanisms of Liver Regeneration
Liver regeneration is the product of tight networking between multiple pathways, but surely the two processes that must be modulated involve cell proliferation and cell growth. They can be defined as two sides of the same coin. Specifically, the IL-6/JAK/STAT-3 pathway is involved in proliferation, while PI3K/PDK1/AKT is responsible for cell growth (Figure 1).
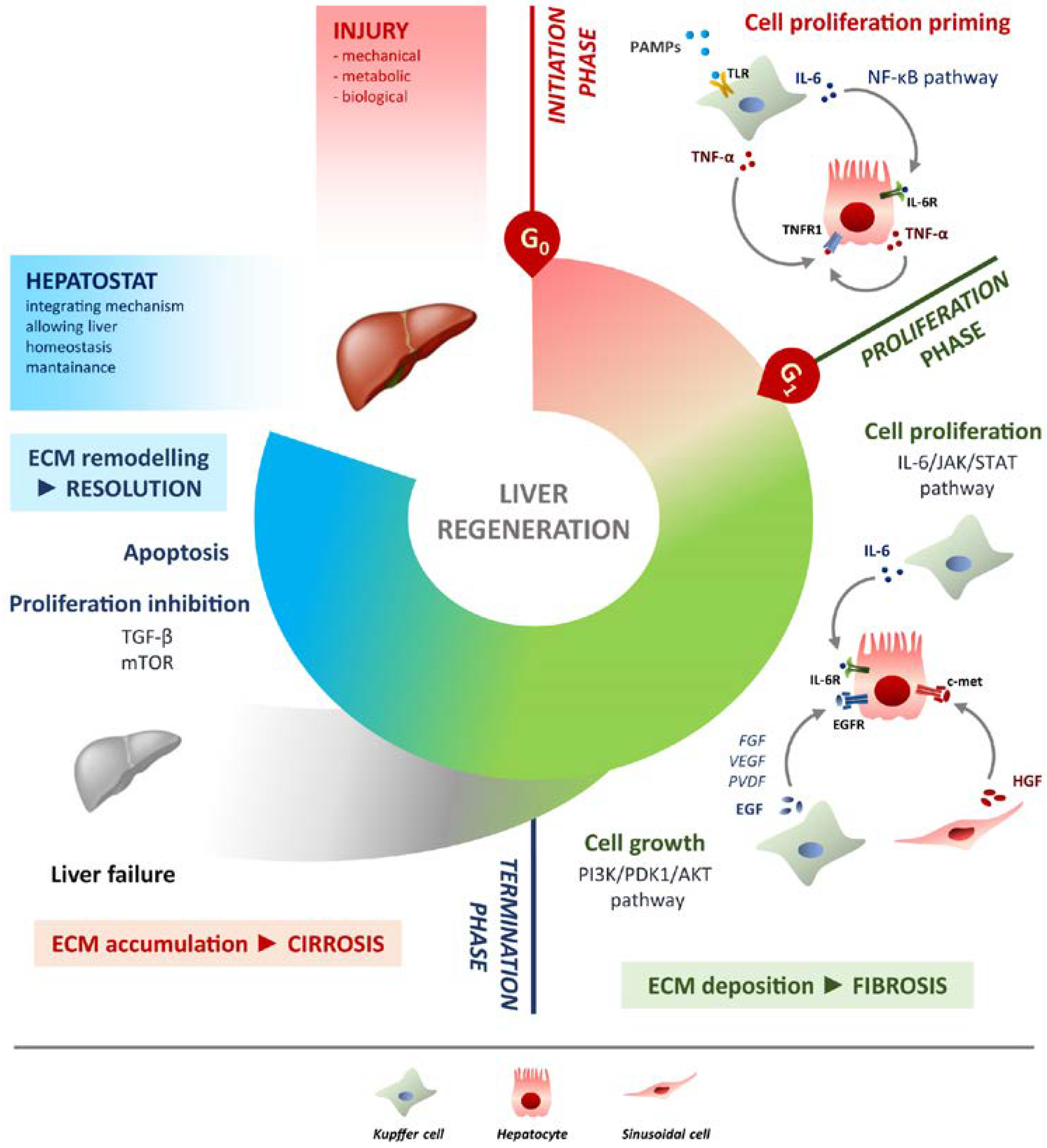
Figure 1. Schematic overview of liver regeneration. LR outlined in three phases: initiation, proliferation and termination. In the initiation phase different PAMPs trigger regenerative process by interacting with TLRs exposed on Kupffer cells membrane. TNF-α and IL-6 produced by Kupffer cells activates the NF-κB pathway in hepatocytes making them responsive to the proliferative signalling and forthcoming to G0/G1 transition. Once IL-6 determined the activation of proliferation, acting on JAK/STAT3 pathway, hepatocytes enter the G1 phase and growth factors signalling ensures their progression in the cell cycle. Kupffer cells and sinusoidal cells sustain hepatocytes growth by producing respectively HGF acting through c-met receptor and EGF. EGFR ligands other than EGF (FGF, VEGF, PDGF and others) act as signal modulators. Growth factors binding to their specific receptors lead to cell growth mainly through the PI3K/PDK1/AKT pathway. The termination phase takes place when the original weight of the liver is reached and results from inhibition of proliferation and growth, and apoptosis induction. The mTOR pathway controls protein translation and together with TGF-β induces hepatocyte expansion inhibition. Apoptosis cooperates with other inhibitory signals, allowing the correct attainment of liver mass. The hepatostat is responsible for the perfect integration of such processes leading liver structural and functional recovery. ECM deposition is a transitory and reversible condition of the regenerative response that arises during the proliferative phase and leads to fibrosis. ECM accumulation requires its rapid and continuous degradation and remodelling to ensure resolution, avoiding cirrhosis outcome and/or liver failure.
IL-17: A Balancer in Liver Regeneration
In recent years, novel molecules involved in LR have been identified, which provide a contribution to the final resolution. Among these, interleukin-17 (IL-17) is certainly the most prominent. The IL-17A, hereinafter IL-17, is the most characterised member of the IL-17 family. This cytokine binds to the receptor for IL-17A (IL-17RA), which dimerizes with IL-17RC, triggering the IL-17 signalling [27]. The activation of this pathway recruits the protein adaptor ACT1, which leads to the activation of relevant pathways such as NF-κB and mitogen-activated protein kinases (MAPK) [28][29]. The cells identified as the first producers of IL-17 are CD4+ Th17. Specifically, Th17 differentiate from naive T cells when influenced by TGF-β, IL-6 and IL-21, which amplify Th17 and induce the expression of the receptor for IL-23 (IL-23R). At this step, IL-23 binds to its receptor and allows the stabilization of the phenotype Th17, which will produce IL-17 [30]. The function of Th17 is offset by regulatory CD4+ T cells (Treg). The presence or absence of inflammation may or may not favour differentiation towards one cell type or the other. Moreover, immune homeostasis also depends on the balance of Th17/Treg and thus on the regulation of the corresponding pathways [31][32][33]. However, Th17s are not the only cells that produce IL-17. In response to different stimuli, other immune cells can produce IL-17, such as γδ T cells, CD8+ T cells and natural killer (NK) cells [34][35]. The pro-inflammatory role of IL-17 is demonstrated in both acute and chronic inflammation. In addition, the activity of IL-17 often requires synergy with other immune molecules. In particular, cooperating with TNF-α, IL-17 induces a massive production of IL-6 and IL-8 in the hepatocytes, which leads to the activation of pro-inflammatory genes [36][37]. The IL-6-promoted inflammatory environment includes neutrophils recruitment and activation of fibroblasts followed by hepatic fibrosis [38][39][40]. IL-17 acts on liver cell types other than hepatocytes. Kupffer cells express IL-17R when stimulated by IL-17 and increase their immunological activity and produce other pro-inflammatory cytokines [41].
4. Conclusions
The study of liver regeneration is clinically relevant to investigate liver transplantation or hepatocarcinoma in human, it also provides a powerful model for studying in vivo the pathways of proliferation and growth. The successful process is guaranteed by the integration of different mechanisms, from the action of specific molecules to well-established timescales. Curiously, the LR is like the Staircase of San Patrizio in Umbria (Italy). The two stairways, up and down, do not cross each other, albeit in acoustic communication, but both serve to reach the same place/target. Likewise, during liver mass restoring, cell proliferation and growth are on one side, sustained by pro-inflammatory immunity, while the remodelling process helped by anti-inflammatory measures is on the other; the equilibrium retains and preserves the physiology and liver function, thus guaranteeing the homeostasis of the organism. The early phases of the regeneration process have certainly been better characterised and it is true that “a good beginning is half the battle”, but in this specific case the terminal phase is equally important. Indeed, the ending of regeneration by the hepatostat after basal values have been restored is a critical point. One of the inhibitory signals could be the activation of the apoptosis process, which would stop cell division and growth. Indeed, to the well-known molecules involved, such as IL-6 and TNF-α, IL-17 undoubtedly covered an important and strategic role in LR. This cytokine is present throughout the regenerative cycle. Effectively, it has been seen as important as contributing to proliferative triggering in the early stages. However, IL-17 role in termination phase is still poorly explored. At this stage, the reduction of IL-17 levels would allow NKT cells to produce INF-γ, thus promoting apoptosis. In the future, the characterisation of IL-17 will be essential. This cytokine is definitely a mediator of inflammation, and its synergetic action with other stimuli makes it crucial in those processes where the action of several mediators is required, such as liver regeneration.
A further contribution in recent years has been made by the development of new in vitro devices, which are proving to be a valid alternative for in vivo research and allow faster characterisation of cell interactions in different biological contexts. These include approaches of both 2D and 3D culture devices, which exploit innovative biomaterials with different properties depending on the type of application. For instance, the use of polymer replicas, such as polycarbonate (PC) or polydimethylsiloxane (PDMS), to which extracellular matrix proteins such as collagen or laminin can be implemented, making the device ideal for studying cellular organisation and differentiation or assessing shear stresses or vascularisation [42][43][44][45][46]. Even more recent are organ-on-a-chip devices, which interest not only the liver, but also other organs and tissues such as heart and kidney [47]. Among the biochips in the hepatic field, it can be found specific devices for the characterisation of pathological conditions such as nonalcoholic fatty liver disease (NAFLD) [48][49][50] or ALD [51][52][53], but also sophisticated microfabricated platforms that allow the assessment of cellular spatial location in accordance with different metabolic levels, which are created ad hoc by applying microfluidics [54].
