Although relatively new in the therapeutic landscape for managing type 2 diabetes (T2D), dipeptidyl-peptidase 4 (DPP4) inhibitors have gained widespread popularity, due to their glycemic efficacy, low risk of hypoglycemic episodes and oral route of administration. DPP4, a cell-bound serine protease abundantly expressed on lymphocytes, epithelial and endothelial cells, plays critical roles in the modulation of glucose homeostasis and inflammatory immune responses. Also, given its potential to serve as an adjunctive functional receptor for the emerging Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) to gain entry into the host, inhibition of DPP4 has been proposed as an alternative and multifaceted strategy to prevent severe clinical manifestations of the SARS-CoV-2 related illness (COVID-19), which are commonly observed in T2D patients. However, the inherent risk of mast cells hyper-activation and the lack of a clear demonstration of SARS-CoV-2 binding to DPP4, may raise some concerns and controversy.
- COVID-19
- type 2 diabetes
- DPP4 inhibitors
- drug repurposing
1. Introduction
Dipeptidyl-peptidase 4 (DPP4) inhibitors, also known as gliptins, represent a relatively new class of oral antidiabetic agents that block the inactivation of the distal gut-derived insulinotropic hormone glucagon-like peptide 1 (GLP1). By positively affecting glucose control with minimal risk of hypoglycemia, these drugs have gained popularity as a second-line option for managing T2D due to their favorable side effect profile and competitive costs [1]. To date, there are five DPP4 inhibitors available on the European market, including sitagliptin, saxagliptin, linagliptin, alogliptin and vildagliptin, all of which have specific pharmacodynamic and pharmacokinetic properties, with potentially relevant implications for patients affected by liver or kidney disease, notwithstanding comparable glycemic efficacy and inhibition of DPP4 activity [2].
2. Potential Benefits and Harms of DPP4 Inhibitors on COVID19 Outcomes
According to mechanistic studies, DPP4, formerly known as T-cell antigen CD26, is a multifunctional soluble and cell-bound serine protease, abundantly expressed in lymphocytes and adipocytes as well as in many other cell types, including endothelial and epithelial cells, which plays critical roles in the modulation of glucose homeostasis and inflammatory responses [1]. Interestingly, DPP4 was identified as a functional receptor for human coronavirus-Erasmus Medical Center (hCoV-EMC) [3], the virus responsible for the Middle East Respiratory Syndrome (MERS) outbreak and genetically close to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which is causing the novel Coronavirus Disease 2019 (COVID-19) pandemic. In addition, antibodies directed against DPP4 could impair hCoV-EMC infection in primary human bronchial epithelial cells. The observation that the S1 domain of SARS-CoV-2 spike glycoprotein interacts with the host receptor protein DPP4 ,[4][5] raised the hypothesis that SARS-CoV-2 may use DPP4 as an adjunctive functional receptor to gain entry into the host, in addition to the well-documented angiotensin-converting enzyme 2 (ACE2) [6]. Although a direct involvement of DPP4 in the modulation of SARS-CoV-2 entry has been denied in a stably transfected human embryonic 293T cell line [7], this computational evidence has raised speculation and questions about whether targeting one of the potential host determinants of virulence with DPP4 inhibitors would be useful in attenuating COVID-19 clinical course after viral exposure [8][9], especially in patients with T2D, typically depicted by a pathogenic dysregulation of DPP4 expression [10], coupled with a hyperglycemia-dependent proinflammatory background [11], and enhanced susceptibility to severe COVID-19 manifestations, including lethality [12]. Apart from the anti-hyperglycemic effect related to protease-dependent modulation of the entero-insular axis, DPP4 inhibitors possess unique immunomodulatory therapeutic potential for autoimmune and rheumatological disorders, cancer and, most remarkably, MERS. Targeting DPP4 to inflect natural viral dynamics has been thus suggested as a pharmacologically reasonable strategy in the case of COVID-19, as well as other severe respiratory diseases related to coronaviruses. Interestingly, rheumatological disorders and severe COVID-19 share the overproduction of NF-kB-dependent proinflammatory cytokines and mediators, such as IL-1, IL-6 and TNF-α, as a common key pathogenetic mechanism [13][14]. The blockade of IL-6 receptors with tocilizumab, a humanized IgG1 monoclonal antibody commonly used in patients with rheumatoid arthritis, is currently under investigation in patients with severe COVID-19 pneumonia, whereas inhibition of IL-1 release by hydroxychloroquine, a first-line disease-modifying antirheumatic drug (DMARD) therapy, has been put under investigation in patients with mild to moderate COVID-19 to prevent disease progression (www.aifa.gov.it). In addition to its immunomodulant and anti-inflammatory effects, hydroxychloroquine has proved to interfere with the glycosylation of SARS-CoV-2 spike proteins and ACE2 receptors, blocking, in in vitro experimental models, viral entry [15]. However, very recently, safety concerns have been raised over its proarrhythmic potential and increased in-hospital death in patients tested positive for SARS-CoV-2 [16], ending in a temporary suspension of clinical trials. It is noteworthy that, during infection progression, SARS-CoV-2 decreases the activity and expression of ACE2, the primary component of an alternate renin-angiotensin system (RAS) that counteracts angiotensin II (ANGII). The unbalanced ANGII activity has been indicated as a critical driver for severe COVID-19 manifestations in lungs and other organs [17]. In this regard, DPP4 inhibitors could interfere with the RASsystem and reduce ANGII levels, thereby ameliorating hypertension and comorbid cardiac remodeling in experimental animal models [18]. However, despite a plausible pharmacological rationale, repurposing drugs with originally different therapeutic indications for preventing or treating COVID-19 can be challenging even in the case of DPP4 inhibitors, as current computational or preclinical evidence does not suffice. To date (May 2020), neither data are available regarding clinical and demographic characteristics of people with diabetes developing severe COVID-19 complications, nor do we know whether background glycemic levels or antidiabetic therapies might have a role. Unfortunately, observational and experimental clinical research programs are comprehensibly more difficult during a pandemic because of many possible barriers, including risk of infection for healthcare workers and research staff deployment to provide clinical care [19]. A metanalysis evidenced that treatment with DPP4 inhibitors does not increase the overall risk of respiratory infections with respect to placebo or conventional oral antidiabetic comparators (metformin, sulfonylureas, thiazolidinediones; OR 0.98, 95% CI 0.91 to 1.05), although no subgroup analysis has been performed concerning relationships with specific viral pathogens [20]. More recently, a large prospective cohort study in France evidenced an inverse association between diabetes and non-influenza respiratory virus illnesses over the course of three flu seasons [21]. The study ended before the COVID-19 pandemic; however, hospitalized patients with flu-like symptoms who tested positive for non-influenza respiratory viruses, including coronavirus 229E, were less likely to have diabetes than patients infected with seasonal influenza viruses (18% vs. 25%). Unfortunately, background information on medication use was not assessed in this study, except for immunosuppressive drugs; therefore, whether specific antidiabetic medications would have prevented non-influenza respiratory virus-related hospitalization in patients with diabetes remains uncertain. Interestingly, and regardless of the viral strain, coronavirus infections are counteracted by submucosal mast cells of the respiratory tract as the forefront part of the innate immunity response [22]. Endothelial transmigration of mast cells is under the control of the stromal derived factor 1 (SDF-1), which is cleaved and inactivated by DPP4 [23][24]. Because of its dose-dependent ability to selectively induce the production of IL-8, but not other pro-inflammatory mediators from mast cells, SDF-1 orchestrates leukocyte recruitment and vascular remodeling in the context of arteriogenesis, both of which could be boosted by DPP4 blockage in experimental animal models [25]. In addition, increased plasmatic levels of SDF-1 have been associated with maturation and migration of leukocytes to mucosal compartments and immune response-dependent abortion of human immunodeficiency virus 1 (HIV1) infection [26]. An intriguing question that could be raised is whether DPP4 inhibitor-related augmentation of SDF-1 levels and mast cells in mucosal entry sites would confer resistance against SARS-CoV-2 and other coronaviruses. In this regard, some evidence suggests that, while neutralizing these pathogens, mast cells release antiviral and cytotoxic mediators which, in some circumstances, can aggravate the clinical course of the disease. The dual effects of mast cells in viral infections could introduce further arguments and controversies in the debate about the use of DPP4 inhibitors as a potential protective strategy against severe COVID-19 manifestations [27]. While downregulation and inhibition of DPP4 activity by hCoV-EMC and HIV1 have been demonstrated [27], to date, there is no indication that binding of SARS-CoV-2 would lead to similar effects. In addition, concerning COVID-19 related vascular damage, it should be noted that, in contrast to preclinical evidence, human studies have shown either a neutral [28] or a detrimental [29] effect of DPP4 blockage on endothelial function in the setting of diabetes.
Figure 1 summarizes the potential benefits and harms of DPP4 inhibitors on COVID-19 outcomes.
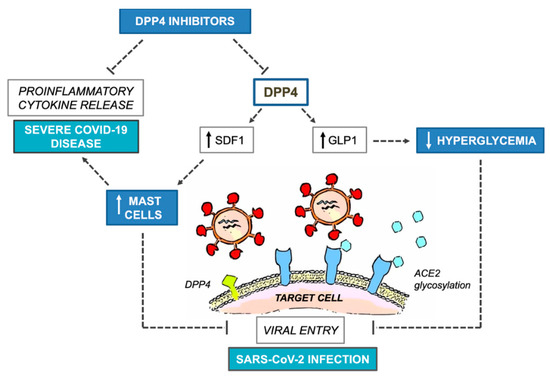
Figure 1. Potential benefits and harms of DPP4 inhibitors on COVID-19 outcomes. COVID-19, Coronavirus Disease 2019; DPP4, dipeptidyl-peptidase 4; SDF1, stromal derived factor 1; GLP1, glucagon-like peptide 1; ACE2, angiotensin converting enzyme 2; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph17103664
References
- Deacon, C.F.; Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front Endocrinol (Lausanne). 2019, 10, 80, 10.3389/fendo.2019.00080..
- Makrilakis, K.; The Role of DPP-4 Inhibitors in the Treatment Algorithm of Type 2 Diabetes Mellitus: When to Select, What to Expect. . Int J Environ Res Public Health. 2019, 16, 2720, 10.3390/ijerph16152720..
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. . Nature 2013, 495, 251–254, 10.1038/nature12005..
- Vankadari, N.; Wilce, J.A.; Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26.. Emerg. Microbes Infect. 2020, 9, 601–604, 10.1080/22221751.2020.1739565..
- Letko, M.; Marzi, A.; Munster, V.; Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses.. Nat. Microbiol. 2020, 5, 562–569, 10.1038/s41564-020-0688-y..
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D; Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. . N. Engl. J. Med 2020, 382, 1653-1659, 10.1056/NEJMoa2008975..
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L.; Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine.. Cell Mol. Immunol. 2020, 8, 0, 10.1038/s41423-020-0400-4..
- Iacobellis, G.; COVID-19 and diabetes: Can DPP4 inhibition play a role?. Diabetes Res. Clin. Pract. 2020, 162, ., 10.1016/j.diabres.2020.108125..
- Fadini, G.P.; Morieri, M.L.; Longato, E.; Avogaro, A.; Prevalence and impact of diabetes among people infected with SARS-CoV-2.. J. Endocrinol. Invest. 2020, 43, 867–869, 10.1007/s40618-020-01236-2..
- Röhrborn, D.; Wronkowitz, N.; Eckel, J.; DPP4 in diabetes.. Front. Immunol. 2015, 6, 386, 10.3389/fimmu.2015.00386..
- Palella, E.; Cimino, R.; Pullano, S.A.; Fiorillo, A.S.; Gulletta, E.; Brunetti, A.; Foti, D.P.; Greco, M.; Laboratory parameters of hemostasis, adhesion molecules, and inflammation in type 2 diabetes mellitus: Correlation with glycemic control.. Int. J. Environ. Res. Public Health. 2020, 17, 300, 10.3390/ijerph17010300..
- Mirabelli, M.; Chiefari, E.; Puccio, L.; Foti, D.P.; Brunetti, A.; Potential Benefits and Harms of Novel Antidiabetic Drugs During COVID-19 Crisis.. Int. J. Environ. Res. Public Health. 2020, 17, 3664, https://doi.org/10.3390/ijerph17103664.
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C.; NF-κB signaling in inflammation.. Signal Transduct. Target Ther. 2017, 2, ., 10.1038/sigtrans.2017.23..
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China.. Clin. Immunol. 2020, 214, ., 10.1016/j.clim.2020.108393..
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H., Li, Y.; Hu, Z.; Zhong, W.; Wang, M.; Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro.. Cell Discov. 2020, 6, 16, 10.1038/s41421-020-0156-0..
- Mehra, M.R.; Desai, S.S.; Ruschitzka, F.; Patel, A.N.; Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis.. Lancet. 2020, ., ., 10.1016/S0140-6736(20)31180-6..
- Sriram, K.; Insel, P.A.; A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance.. Br. J. Pharmacol. 2020, 37, ., 10.1111/bph.15082..
- Kawase, H.; Bando, Y.K.; Nishimura, K.; Aoyama, M.; Monji, A.; Murohara, T.; A dipeptidyl peptidase-4 inhibitor ameliorates hypertensive cardiac remodeling via angiotensin-II/sodium-proton pump exchanger-1 axis.. J Mol Cell Cardiol. 2016, 98, 37–47, 10.1016/j.yjmcc.2016.06.066..
- Cook, D.J.; Marshall, J.C.; Fowler, R.A.; Critical Illness in Patients With COVID-19 Mounting an Effective Clinical and Research Response.. JAMA 2020, 323, 1559-1560, 10.1001/jama.2020.5775..
- Yang, W.; Cai, X.; Han, X.; Ji, L.; DPP-4 inhibitors and risk of infections: A meta-analysis of randomized controlled trials.. Diabetes Metab. Res. Rev. 2016, 32, 391–404, 10.1002/dmrr.2723..
- Bénézit, F.; Loubet, P.; Galtier, F.; Pronier, C.; Lenzi, N.; Lesieur, Z.; Jouneau, S.; Lagathu, G.; L’Honneur, A.S.; Foulongne, V.; et al. FLUVAC Study Group. Non-influenza respiratory viruses in adult patients admitted with influenza-like illness: A 3-year prospective multicenter study.. Infection. 2020, 13, 1-7, 10.1007/s15010-019-01388-1.
- Kritas, S.K.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Conti, P.; 27. Kritas, S.K.; Ronconi, G.; Caraffa, AMast cells contribute to coronavirus-induced inflammation: New anti-inflammatory strategy.; Gallenga, C.E.; Ross, R.; Conti, P.. J. Biol. Regul. Homeost. Agents 2020, 34, 1, 10.23812/20-editorial-kritas .
- Lin, T.J.; Issekutz, T.B.; Marshall, J.S.; SDF-1 induces IL-8 production and transendothelial migration of human cord blood-derived mast cells. . Int. Arch. Allergy Immunol. 2001, 124, 142–145, 10.1159/000053693.
- Vedantham, S.; Kluever, A.K.; Deindl, E.; Is there a Chance to Promote Arteriogenesis by DPP4 Inhibitors Even in Type 2 Diabetes? A Critical Review.. Cells 2018, 7, 181, 10.3390/cells7100181.
- Chillo, O.; Kleinert, E.C.; Lautz, T.; Lasch, M.; Pagel, J.I.; Heun, Y.; Troidl, K.; Fischer, S.; Caballero-Martinez, A.; Mauer, A.; et al. Perivascular Mast Cells Govern Shear Stress-Induced Arteriogenesis by Orchestrating Leukocyte Function. . Cell Rep. 2016, 23, 2197-2207, 10.1016/j.celrep.2016.07.040..
- Soriano, A.; Martínez, C.; García, F.; Plana, M.; Palou, E.; Lejeune, M.; Aróstegui, J.I.; De Lazzari, E.; Rodriguez, C.; Barrasa, A.; et al. Plasma stromal cell-derived factor (SDF)-1 levels, SDF1-3’A genotype, and expression of CXCR4 on T lymphocytes: Their impact on resistance to human immunodeficiency virus type 1 infection and its progression.. J. Infect. Dis. 2002, 186, 922–931, 10.1086/343741.
- Pitocco, D.; Tartaglione, L.; Viti, L.; Di Leo, M.; Pontecorvi, A.; Caputo, S.; SARS-CoV-2 and DPP4 inhibition: Is it time to pray for Janus Bifrons?. Diabetes Res. Clin. Pract. 2020, 163, ., 10.1016/j.diabres.2020.108162.
- Widlansky, M.E.; Puppala, V.K.; Suboc, T.M.; Malik, M.; Branum, A.; Signorelli, K.; Wang, J.; Ying, R.; Tanner, M.J.; Tyagi, S.; et al. Impact of DPP-4 inhibition on acute and chronic endothelial function in humans with type 2 diabetes on background metformin therapy.. Vasc. Med. 2017, 22, 189–196, 10.1177/1358863X16681486..
- Ayaori, M.; Iwakami, N.; Uto-Kondo, H.; Sato, H.; Sasaki, M.; Komatsu, T.; Iizuka, M.; Takiguchi, S.; Yakushiji, E.; Nakaya, K.; et al. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. . J. Am. Heart Assoc. 2013, 2, ., 10.1161/JAHA.112.003277..
