However, dysregulated expression of Fli1 due to genetic mutations was recognized as one of the causative factors leading to malignant transformation of cells and the progression of multiple hematological and solid cancers [
13], including Ewing’s sarcoma [
14] and prostate cancer [
15]. Moreover, over the past decades Fli1 has attracted great attention for its role in the etiology of several autoimmune diseases. Sustained Fli1 downregulation is associated with the development of systemic sclerosis, while overexpression of the
FLI1 gene is a well-acknowledged event in systemic lupus erythematosus [
3]. Abnormal Fli1 activity is implicated in the development of hypertensive disorders such as uremic cardiomyopathy [
16] and preeclampsia [
17]. Next, Fli1 deficiency was identified as an essential component of the signaling pathways underlying pro-fibrotic processes, a common phenomenon accompanying the progression of many diseases. Experiments using genetically engineered rodents, in vitro
FLI1 gene silencing, dominant interference, and DNA binding mutants have indicated that Fli1 inhibition is mediated by both direct (DNA binding) and indirect (protein–protein interaction) mechanisms [
8,
12,
18]. Over the past few years, a new trend in investigations increasingly reports the genetic predisposition and epigenetic modifications of genes related to Fli1-dependent pathologies. Besides genetics, epigenetics has been suggested as playing a pivotal role in the development and clinical manifestations of diseases [
19,
20,
21]. Aberrant expression of Fli1 might be responsible for disturbed functioning of a series of genes including that encoding integrins, growth factor receptors, chemokines, and adhesion molecules.
2. Diseases Associated with Fli1 Deficiency
2.1. Fli1 in Systemic Sclerosis
Fli1 as inductor of fibrosis. Systemic sclerosis (SSc or scleroderma) is a complex and highly heterogeneous autoimmune disease starting from dysregulation of the immune system and inflammation and followed by impaired angiogenesis, widespread vascular injury, and blood coagulation defects [
22]. Persistently activated interstitial fibroblasts induce irreversible fibrosis of the skin and multiple internal organs. Except skin lesions, SSc is accompanied by cardiac defects (lesions in the coronary arteries, pericardium, and myocardium, and myocardial fibrosis) and renal failure (vascular lesions and fibrosis in the kidney, damage to renal glomeruli, and impaired glomerular filtration).
Although the etiology of this life-threatening disorder is not completely understood, SSc-associated vasculopathy was demonstrated to result from hyperactivation of vascular endothelial cells due to genetic or environmental factors leading to dysregulated vascular remodeling. The central mechanism underlying tissue fibrosis is excessive deposition of extracellular matrix (ECM) proteins and impaired collagen homeostasis. Activated interstitial fibroblasts and immune cells synthesize excessive amounts of collagens (first of all types I, III, VI, and VII) and other ECM components such as fibronectin, tenascin-C (TNC), and alpha smooth muscle actin (αSMA), profibrotic cytokines including TGF-β and connective tissue growth factor CTGF (also known as CCN2), accompanied by reduced expression of ECM-degrading enzymes MMP (matrix metalloproteinases) 1 and 3 [
23,
24].
The causative relationship between transcription factors from the ETS family and pro-fibrotic processes was first established in the pioneering work of Czuwara-Ladykowska et al. [
25], which demonstrated that Fli1 plays a crucial role in the regulation of collagen synthesis in dermal fibroblasts by competing with Ets-1, and the ratio of Fli1 to Ets-1 in the presence of co-regulatory proteins may control collagen production. Further in vitro studies have confirmed that
FLI1 suppression induces SSc-like phenotypes in dermal fibroblasts, dermal dendritic cells, keratinocytes, endothelial cells, and macrophages, while in vivo findings have established that Fli1 deficiency is an important mediator of SSc-associated fibrosis [
26,
27,
28]. The role of Fli1 as a natural negative inhibitor of collagen genes was supported by the fact that Fli1 protein levels were inversely proportional with COL1A1 mRNA and collagen 1 levels in the dermal fibroblasts of patients with SSc and in cultured fibroblast isolated from Fli1−/−, Fli1+/−, and Fli1+/+ mice embryos [
29]. In the lesional and non-lesional skin of SSc patients, Fli1-positive fibroblasts are either consistently absent or only occasionally seen, and Fli1 immunoreactivity is significantly reduced in endothelial cells [
29]. Fli1 is downregulated in dermal dendritic cells [
30] and in the primary skin fibroblasts of SSc patients [
31]. Furthermore, Fli1 has been demonstrated to play an important role in the regulation of other ECM proteins’ synthesis, including MMP-1 and CTGF [
32,
33]. Fli1 deficiency impairs the expression of various genes both in fibroblasts and endothelial cells and may represent a unique pathogenetic link between dermal fibrosis and peripheral vasculopathy in SSc. The critical role of Fli1 in the development of this disease was confirmed in the works on
FLI1-mutated animals such as Fli1+/− mice [
8] or that with a conditional deletion of
FLI1 in endothelial cells (Fli1 CKO mice) [
34].
Fli1-DNA binding. Under normal physiological conditions, Fli1 and Ets-1 form DNA–protein complexes with the sequences of the
COL1A2 gene promoter. The suppressive interaction of Fli1with
COL1 gene is functionally enhanced by co-regulating factors such as Sp1 and Sp3 [
25]. Stable
FLI1 transfection leads to dramatic suppression of COL1A2 mRNA and newly synthesized collagenous proteins by inhibiting
COL1A2 promoter activity, while Ets-1 activates the
COL1A2 promoter, which indicates that Fli1 inhibits the
COL1A2 gene by displacing Ets-1. Recent studies have suggested that Fli1 also binds to promoters of genes encoding other proteins such as CCN2 [
32], pro-angiogenic adipokine chemerin involved in inflammation, angiogenesis and energy metabolism [
35], and one of the damage-associated molecular patterns (DAMPs) molecules S100A12 [
36].
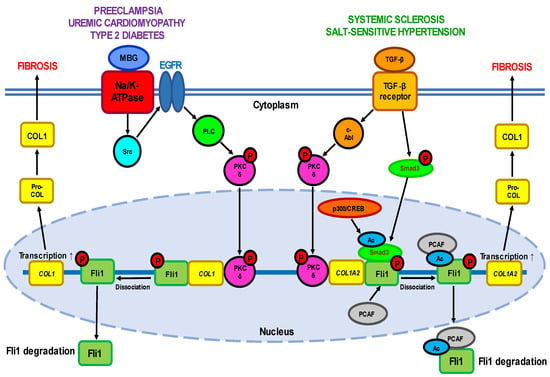
Fli1 signaling pathways. The transcriptional potential of Fli1 is strictly regulated by a balance between the activities of two cytokines—pro-fibrotic TGF-β, a well-established inductor of ECM proteins’ synthesis, and anti-fibrotic TNFα which prevents uncontrolled ECM production. TGF-β stimulation leads to activation of a few cellular targets (
Figure 1,
Table 1). Active PKC-δ is translocated to the nucleus and recruited to the
COL1A2 promoter, where it directly phosphorylates Fli1 at threonine 312 [
37]. Phosphorylation at this residue is necessary for interaction of Fli1 with p300/CREB-binding protein-associated factor (PCAF) resulting in acetylation of Fli1 at lysine 380 [
38,
39]. Acetylated Fli1 dissociates from the
COL1A2 promoter and degrades, while decreased Fli1 transcription enhances the expression of
COL genes. Another downstream effector of TGF-β is nonreceptor kinase c-Abl which activates the nuclear translocation of PKC-δ thus facilitating Fli1 acetylation and decreasing Fli1 repression of the
COL1A2 gene [
40]. Indeed, persistent stimulation of the c-Abl/PKC-δ/Fli1 cascade at least partially implicates in the fibrotic program in dermal fibroblasts obtained from patients with both SSc and its relative disease—localized scleroderma (LSc) [
41]. Moreover, the c-Abl–PKC-δ–Fli1 pathway is activated by TGF-β-dependent stimulation of endothelin-1 [
42].
Figure 1. A simplified scheme of the Fli1-dependent pro-fibrotic pathways described for different diseases. One of the pathways is induced by interaction of active TGF-β with its plasma membrane receptor and depends on the ratio between the COL1-suppressive activity of Fli1 and the stimulatory ability of Smad3. TGF-β-dependent activation leads to phosphorylation of c-Abl which in turn induces nuclear translocation of PKC-δ. In the nucleus, PKC-δ phosphorylates Fli1 at threonine 312 thus facilitating the acetylation of Fli1 at lysine 380 by PCAF (p300/CREB-binding protein-associated factor). These events result in dissociation of Fli1 from promoter of the COL1A2 gene and its rapid proteolysis, followed by increased transcription of the COL1 gene. On the other hand, activation of TGF-β receptor phosphorylates Smad3 which moves into the nucleus and undergoes acetylation by the p300/CREB-binding protein which increases its DNA binding capacity with the promoter of the COL1A2 gene. The second pathway is triggered by binding of MBG to the plasma membrane Na-K-ATPase, which results in phosphorylation of tyrosine kinase Src followed by sequential phosphorylation/stimulation of PLC and PKC-δ. Active PKC-δ is translocated to the nucleus where it disrupts Fli1 repression of the COL1 gene leading to the loss of Fli1 activity and excessive collagen synthesis.
A variety of other factors contribute to SSc due to aberrant expression of Fli1 (
Table 1). Among them are cathepsins, proteolytic enzymes regulating angiogenesis and ECM degradation. Enhanced expression of endothelial cathepsin B and cathepsin L due to Fli1 deficiency has been shown to be associated with SSc vasculopathy, whereas downregulation of CTSB, CTSL, and CTSV in dermal fibroblasts implicates in SSc dermal fibrosis [
43,
44,
45]. Next, Fli1 acts as a repressor of CTGF growth factor, an important regulator of angiogenesis, chondrogenesis, and wound healing. Downregulation of Fli1 upregulated the expression of CTGF mRNA which mimics to some extent the profibrotic effects of transforming growth factor TGF-β such as the upregulation of the
COL1A1 and
COL1A2 genes and the downregulation of the
MMP1 gene [
32]. Fli1 is a strong inhibitor of a pleiotropic growth factor progranulin (PGRN), an endogenous antagonist of tumor necrosis factor (TNF) receptors, and Fli1 suppression was shown to be associated with upregulated expression of PGRN and TNF-α in the skin of dsSSc patients, Fli1+/− mice, and bleomycin (BLM)-treated mice [
46,
47].
Besides, Fli1 was shown to regulate the activity of a few angiogenesis-related chemokines with pro- or anti-angiogenic properties in the context of SSc-associated vasculopathy. For instance, Fli1 deficiency is associated with suppression of CXCL5 in the dermal blood vessels of SSc patients, Fli1 siRNA transfected endothelial cells, and Fli1 knockout mice [
48]. In contrast, the progression of vasculopathy and fibrosis in SSc heart, lung, and skin due to Fli1 deficiency in fibroblasts and endothelial cells was accompanied by upregulation of CXCL6 [
49], while CXCL13 (a chemokine for B cells, follicular T cells, T helper 17 cells, and regulatory T cells) expression was enhanced by Fli1 deficiency in peritoneal murine macrophages [
50]. CXCL4, a chemokine with anti-angiogenic capacity, may contribute to peripheral SSc vasculopathy by downregulating Fli1 via c-Abl signaling in endothelial cells. Furthermore, CXCL4 blocked the cell proliferation of HUVECs induced by TGF-β or platelet-derived growth factor (PDGF) [
51]. Using three experimental models, namely skin biopsy samples of SSc patients, cultured human dermal microvascular endothelial cells (HDMECs), and a BLM-induced mice SSc model of skin fibrosis, Ikawa et al. [
52] showed that the development of SSc vasculopathy is associated with increased expression of endothelial CCR6 due to Fli1 deficiency in dermal small vessels irrespective of disease subtypes and disease duration, while CCL20 expression was significantly elevated in dermal fibroblasts of patients with early dcSSc.
Fli1 has also been demonstrated to be implicated in the regulation of activities of a wide variety of genes involved in different aspects of cell physiology. Fli1 deletion altered the expression of the proteins involved in vascular homeostasis including VE-cadherin, platelet endothelial cell adhesion molecule 1 (PECAM-1), MMP-9, platelet-derived growth factor B (PDGFB), and S1P(1) receptor in human, Fli1+/− mice, and Fli1 CKO mice dermal microvascular endothelial cells [
34]. Fli1 deficiency was suggested to contribute to the suppression of RALDH1 (aldehyde dehydrogenase 1 family member A1) in the dermal dendritic cells (DCs) of patients with diffuse cutaneous SSc and BLM-treated Fli1+/− mice [
30]. Another Fli1 target is pro-angiogenic adipokine chemerin, which is involved in inflammation, adipogenesis, angiogenesis, and energy metabolism. The silencing of Fli1, which binds to the chemerin promoter, induced chemerin expression in HDMECs, while Fli1+/− mice exhibited elevated chemerin expression in dermal blood vessels [
35]. Fli1 deficiency was shown to be linked with elevated expression of calcium-binding protein S100A12 at the protein level in the epidermis of SSc-involved skin and at the mRNA level in the bulk skin [
36]. Hypercoagulation conditions associated with tissue fibrosis and impaired peripheral circulation in SSc was shown to result from Fli1-dependent downregulation of endothelial protein C receptor (EPCR) playing a critical role in the regulation of coagulation system and mediating various cytoprotective effects [
53]. The development of SSc-associated pulmonary vascular hypertension was suggested to be linked with Fli1-deficiency-induced upregulation of adipsin, serine proteinase catalyzing the breakdown of complement factor C3 [
54].
Genetic and epigenetic modulations. During the last few years, SSc development has been shown to greatly depend on the complex interaction between genetic predisposition which determines the susceptibility to SSc and epigenetic modifications which modulate the severity of disease, especially pro-fibrotic processes.
FLI1 is genetically and epigenetically suppressed in SSc patients, suggesting Fli1 deficiency as an important factor in SSc etiology [
19,
20,
21,
55]. Fli1+/− mice exhibit an enhanced deposition of type I collagen in the skin and develop mild vascular lesions, while BLM-treated Fli1+/− mice develop skin fibrosis and vascular pathologies [
8]. However, the exact mutations associated with SSc have virtually not been explored. The only human genetic variant found to influence Fli1 expression is the polymorphism of (GA)n (GA dinucleotide repeat sequence) microsatellites located at 5′ UTR of the
FLI1 gene [
56]. Patients possessing (GA)n alleles with ≥ 22 repeats exhibit decreased Fli1 mRNA levels in the peripheral blood but greater susceptibility to both diffuse cutaneous and limited cutaneous SSc in comparison to healthy controls with (GA)n alleles ≤21.
Growing evidence has suggested that not only inheritance but also epigenetic modifications can mediate the emergence and persistence of SSc phenotypes (
Table 1). Fli1 was shown to be a target of all known epigenetic mechanisms, from DNA methylation and histone acetylation to non-coding RNA (microRNA and long non-coding RNA). Thus, compared with the controls, skin biopsy specimens and fibroblasts of SSc patients exhibited hypermethylation of CpG islands and reduced acetylation of histones H3 and H4 in the promoter region of the
FLI1 gene, as well as significantly higher levels of DNA methyltransferase DNMT1, histone deacetylases HDAC1 and HDAC6, proteins MBD-1 and MBD-2 (proteins containing a methyl-CpG DNA binding domain), and MeCP2 (protein of the methylated CpG binding domain) [
57]. Genome-wide DNA methylation analysis of skin fibroblast from patients with diffuse and limited cutaneous SSc demonstrated a substantial number of hypomethylated genes relative to ECM proteins’ turnover, including a series of collagen genes (
COL23A1,
COL4A2,
COL8A1,
COL16A1,
COL29A1,
COL1A1,
COL6A3, and
COL12A1),
PAX9 encoding the pro-α2 chain of type I collagen,
ITGA9 encoding α integrin,
TNXB encoding tenascin-XB,
ADAM12 encoding metallopeptidase, and transcription factors gene
RUNX1-3 [
58].
Except chromatin remodeling, a variety of miRNAs regulating cell grow differentiation and immune functions have been shown to be implicated in SSc-dependent fibrotic processes. Knockdown of pro-fibrotic miR-26a, which was predicted to target the
FLI1 untranslated region, increased Fli1 expression and decreased collagen I and fibronectin expression in SSc skin fibroblasts, while miR-26 transfection reduced the Fli1 level and enhanced collagen I and fibronectin accumulation [
31]. The synthesis of collagen has also been shown to be regulated by a series of miRNAs with pro- or anti-fibrotic properties. Among the pro-fibrotic ones is miR-21, whose expression is upregulated in both dcSSc- and TGF-β-treated fibroblasts [
59]. Anti-fibrotic miR-29a, known to bind the 3′ UTR of the
COL1A1 gene, modulates collagen production in an opposing way. Its level was significantly decreased in SSc fibroblasts, SSc skin biopsies, and SSc skin samples from BLM-treated mice, while its overexpression in SSc fibroblasts suppressed collagen synthesis [
59,
60]. Other anti-fibrotic miRNAs, miR-196a and miR-let-7a, were found to be downregulated both in dermal fibroblasts and the skin of SSc patients thus resulting in abnormal collagen expression [
61,
62]. In addition, collagen production in SSc fibroblasts was enhanced by the suppression of miR-135b, while the serum and monocytes of SSc patients exhibited diminished levels of miR-135b due to methylation [
63].
2.2. Fli1 in Uremic Cardiomyopathy
Uremic cardiomyopathy (UC) is a multifactorial pathological condition developed in the patients with chronic kidney disease (CKD) and is responsible for increased rates of heart failure and high mortality, particularly sudden cardiac death [
64]. It is important that the risk of developing cardiovascular defects is even higher than that of kidney failure. UC is characterized by remodeling of cardiac tissues leading to hypertrophy of left ventricle and diastolic dysfunction, and by a myriad of metabolic maladaptations in myocardial cells including disturbed mitochondrial function, changes in substrate utilization and metabolic transporter function, and altered insulin response.
The attempts to understand the cardiac perturbations accompanying CKD have revealed its link with abnormal Fli1 expression and increased collagen production as well (
Figure 1). Early work has demonstrated that Fli1 transgenic mice developed a high incidence of immunological renal disease and ultimately died of renal failure caused by tubulointerstitial nephritis and immune-complex glomerulonephritis [
65]. Later studies have strongly implicated the transcription factor Fli1 in the progressive cardiac fibrosis seen with experimental uremia. The works on rats that underwent partial nephrectomy have shown that many of the clinical features of experimental UC are accompanied by an increase in circulating concentrations of marinobufagenin (MBG), one of the cardiotonic steroids [
66,
67]. Moreover, MBG signaling through Na/K-ATPase is directly responsible for suppression of Fli1 and the stimulation of cardiac fibroblasts to produce increased amounts of collagen, thus triggering cardiac fibrosis seen with experimental renal failure [
66,
67]. These findings were confirmed by the fact that intraperitoneal administration of monoclonal antibodies to MBG restored Fli1 expression and reduced cardiac fibrosis [
68]. Isolated cardiac fibroblasts cultured with 1 nM MBG in vitro increased procollagen-1 expression accompanied by synthesis of procollagen-1 mRNA and collagen translation [
67]. The stimulation of fibroblasts with MBG was prevented by treatment with inhibitors of tyrosine phosphorylation, Src activation, EGFR transactivation, and N-acetyl cysteine. In general, the signaling pathway leading from MBG to increased collagen synthesis resembles that observed in other Fli1-deficiency-associated diseases such as SSc including translocation of PKC-δ to the nucleus where it stimulates Fli1 [
16].
2.3. Fli1 in Preeclampsia
Another example of pathology at least partially associated with aberrantly low Fli1 expression and the development of vascular fibrosis is preeclampsia (PE), a hypertensive complication of pregnancy adversely affecting both mothers and newborns and in some cases leading to maternal stroke and death [
17,
69,
70]. PE is believed to depend on pre-existing cardiovascular abnormalities and is associated with a life-long risk of hypertension and coronary artery disease [
70]. Among others, SARS-CoV-2 infection has recently been suggested to be linked with higher PE incidence, most probably due to inflammatory responses which can infect the placenta and impair its normal development [
71]. An important role in PE belongs to maternal and placental vascular dysfunction, in particular abnormal remodeling of spiral arteries, endothelial damage, and an imbalance between the release of pro- and antiangiogenic factors (such as vascular endothelial growth factor A VEGFA, placental growth factor PlGF, soluble fms-like tyrosine kinase 1 sFLT-1, endoglin ENG, and soluble sENG) [
72].
On the other hand, the progression of PE is closely related to enhanced synthesis of MBG and the development of cardiovascular fibrosis involving Fli1-dependent mechanisms [
17,
69]. Clinical studies have demonstrated that in umbilical arteries and placentas obtained from PE patients, the expression of Fli1 was significantly (4–9-fold) diminished, while the levels of pro-collagen and collagen-1 increased (2.5–3-fold) [
73,
74,
75]. Similar results were obtained in the work on pregnant Sprague Dawley rats in which PE was induced experimentally by exposure to water with excessive NaCl amounts (1.8 %). The development of a PE-like phenotype in animals was accompanied by a seven-fold decrease in Fli1 expression and a four-fold increase in collagen-1 synthesis in thoracic aortas [
76]. Finally, silencing of the
FLI1 gene by 24 h incubation of the isolated segment of healthy human umbilical arteries with Fli1 siRNA not only suppressed the expression of Fli1 at the protein level, but also led to pronounced elevation of pro-collagen-1 and collagen-1 levels [
77].
A causative relationship between MBG and Fli1 was confirmed in experiments in vitro on explants of umbilical arteries from healthy women. Incubation of the vascular fragments with 1-10 nM MBG for 24–48 h resulted in a considerable (4-5-fold) decrease in Fli1 levels and accumulation (2–3-fold) of pro-collagen-1 and collagen-1, thus mimicking the processes typical for PE and linked with vascular fibrosis [
73,
74,
75]. In contrast, the degree of collagen-1 accumulation was diminished after the pre-treatment of arterial rings from normotensive pregnant women with monoclonal anti-MBG antibodies [
74]. Application of canrenone, an active metabolite of spironolactone used in hypertensive therapy, fully restored Fli1 expression and partially suppressed collagen-1 synthesis in umbilical arteries from PE patients and in the vessels of healthy women incubated with MBG [
75]. Moreover, short-term administration of anti-MBG antibodies to rats with experimental PE reversed Fli1 inhibition, although it had no effect on collagen-1 synthesis [
76]. However, the underlying molecular pathways of PE in human or rat vessels were not associated with the stimulation of TGF-β and Smad proteins (
Figure 1), although the protein level of cytoplasmic PKC-δ increased.
2.4. Fli1 as a Common Causative Factor of Hypertension
Interestingly, the patients with preeclampsia [
17], chronic renal failure [
68,
78], and type 2 diabetes [
79] shared the same phenotype features, namely the development of cardiovascular fibrosis due to Fli1 deficiency. Researchers assume that the etiology and underlying molecular mechanisms of all these disorders are common and at least in part include enhanced levels of circulating MBG and inhibition of membrane enzyme Na/K-ATPase. The phenomena of MBG-induced fibrosis are sensitive to pharmacological modulation of Na/K-ATPase activity with anti-hypertensive agents [
75]. The binding of MBG to Na/K-ATPase initiates the signal transduction that complexes with Src and epidermal growth factor receptor (EGFR) that leads to translocation of PKC-δ to the nucleus and Fli1 repression [
16]. However, this process does not involve TGF-β—Smad proteins cascade, although TGF-β antagonist SB-431542 suppressed the stimulation of collagen production [
67]. In contrast, Dahl salt-sensitive rats [
80] and normotensive salt-loaded Sprague Dawley rats [
81] react to a high-salt diet with fibrotic changes in myocardial and renal tissue which are accompanied by the activation of TGF-β [
81]. Although the association of MBG and TGF-β described in these experimental models is clear, the causative relationship between them in salt sensitivity has not been explored in details. Interestingly, the crosstalk between Fli1 and TGF-β profibrotic signaling via PKC-δ has previously been described, for example, in type 2 diabetes during salt loading [
79]. The development of diabetes is associated with profound inhibition of Fli1 in vascular tissues, while exposure of these rats to high salt levels activates TGF-β and suppresses the expression of Fli1 [
79].
Table 1. Summary of Fli1-associated pro-fibrotic effects.
| Expression |
Effect |
Experimental System |
References |
| Fli1 repressors |
|
|
|
|
| TGF-β |
↑ |
Activation of
TGF-β receptors |
Human dermal fibroblasts;
rat diabetes model;
rat hypertension model |
[25,29,32,38,39,79,80,81] |
| c-Abl |
↑ |
PKC-δ phosphorylation |
Human normal and SSc dermal fibroblasts |
[40,41,47,51] |
| PKC-δ |
↑ |
Fli1 phosphorylation |
Human normal and SSc dermal fibroblasts;
FLI1-transfected HEK293T cells;
FLI1 silencing in human UA |
[37,39,42,47,77] |
| Endothelin-1 |
↑ |
Fli1 activation |
Human normal and SSc dermal fibroblasts;
BLM-treated mice |
[42] |
| PCAF |
↑ |
Fli1 acetylation |
Human dermal fibroblasts;
FLI1-transfected HEK293T cells |
[38] |
| CXCL4 |
↑ |
Vasculopathy |
HUVECs |
[51] |
| MBG |
↑ |
PKC-δ activation; Fli1 suppression |
Wild-type and FLI1-knockdown mice;
rat CKD model;
rat cardiac fibroblasts;
human cardiac fibroblasts;
human renal fibroblasts;
FLI1-transfected renal fibroblasts;
rat diabetes model;
rat hypertension model;
rat PE model |
[16,66,67,68,73,74,75,76,77,79,80,81] |
| Fli1-deficiency targets |
|
| COL1 |
↑ |
Collagen synthesis |
FLI1-transfected human dermal fibroblasts;
human normal and SSc dermal fibroblasts;
murine Fli11−/−, Fli1+/− and Fli1+/+ fibroblasts;
wild-type and FLI1-knockdown mice;
rat CKD model;
rat cardiac fibroblasts;
human cardiac fibroblasts;
human renal fibroblasts;
FLI1-transfected renal fibroblasts;
rat diabetes model;
rat hypertension model;
rat PE model;
human PE UA;
FLI1 silencing in human UA;
human MBG-treated UA |
[8,16,25,29,32,38,66,67,68,73,74,75,76,77,79,80,81] |
| CTGF(CCN2) |
↑ |
COL1A1 and COL1A2 upregulation; MMP-1 downregulation |
FLI1-transfected human dermal fibroblasts;
human SSc fibroblasts |
[32,33] |
| MMP-1 |
↓ |
Increased production of EMC components |
FLI1-transfected human dermal fibroblasts;
human SSc fibroblasts |
[32,33] |
| Fibronectin |
↑ |
Fibrosis |
Human CD14+ monocytes and CD14+ macrophages |
[23] |
| Chemerin |
↑ |
Impaired angiogenesis |
Human SSc dermal vessels and HDMECs;
murine Fli1+/− dermal blood vessels; |
[35] |
| S100A12 |
↑ |
Skin sclerosis |
Human SSc skin; |
[36] |
| RALDH1 |
↓ |
Fibrosis |
Human dermal dendritic cells;
BLM-treated Fli1+/− mice |
[30] |
| Cathepsin B |
↑
↓ |
Vasculopathy
Increased production of EMC components |
FLI1 silencing in HDMECs;
Fli1+/− mice;
human dcSSc dermal fibroblasts, early dcSSc dermal fibroblasts |
[43] |
| Cathepsin L |
↑
↓ |
Vasculopathy
Increased production of EMC components |
FLI1 silencing in HDMECs;
human SS dermal vessels and skin;
FLI1-knockout mice;
skin of BLM-treated mice |
[45] |
| Cathepsin V |
↓ |
Increased production of EMC components |
FLI1 silencing in HDMECs;
human dcSSc dermal fibroblasts, microvascular ECs, dcSSc and lcSSc skin keratinocytes |
[44] |
| PGRN |
↑ |
Inflammation;
skin sclerosis;
fibrosis |
Human SSc dermal fibroblasts;
Fli-1+/− mice;
BLM-treated mice;
human LSc skin lesions |
[46,47] |
| TNF-α |
↑ |
Inflammation;
fibrosis |
Human LSc skin lesions; |
[47] |
| CXCL5 |
↓ |
Vasculopathy |
Human dcSSc dermal blood vessels;
FLI1 silencing in HDMECs;
dermal vessels of Fli1 knockout mice |
[48] |
| CXCL6 |
↑ |
Tissues fibrosis;
vasculopathy |
Human SSc dermal fibroblasts;
FLI1 silencing in HDMECs |
[49] |
| CCL20 |
↑ |
Fibrosis |
Human early dcSSc |
[52] |
| CCL6 |
↑ |
Vasculopathy |
Human SSc dermal vessels;
FLI1 silencing in HDMECs |
[52] |
| EPCR |
↓ |
Impaired vascular homeostasis |
Human SSc dermal vessels;
Fli1+/− mice;
FLI1 silencing in HDMECs |
[53] |
| VE-cadherin |
↓ |
Impaired vascular homeostasis |
MDMECs of Fli1 CKO mice;
MDMECs of Fli1+/− mice;
FLI1 silencing in HDMECs |
[34] |
| PECAM-1 |
↓ |
Impaired vascular homeostasis |
MDMECs of Fli1 CKO mice;
MDMECs of Fli1+/− mice;
FLI1 silencing in HDMECs |
[34] |
| MMP-9 |
↓ |
Impaired vascular homeostasis |
MDMECs of Fli1 CKO mice;
MDMECs of Fli1+/− mice;
FLI1 silencing in HDMECs |
[34] |
| PDGF-B |
↓ |
Impaired vascular homeostasis |
MDMECs of Fli1 CKO mice;
MDMECs of Fli1+/− mice;
FLI1 silencing in HDMECs |
[34] |
| S1P1 |
↓ |
Impaired vascular homeostasis |
MDMECs of Fli1 CKO mice;
MDMECs of Fli1+/− mice;
FLI1 silencing in HDMECs |
[34] |
| Adipsin |
↑ |
Vascular hypertension |
Human SSc dermal vessels;
FLI1 silencing in HDMECs |
[54] |
| Genetic and epigenetic factors |
|
| (GA)n alleles |
↑ |
Increased susceptibility to SSc |
Human SSc peripheral blood |
[56] |
| Acetylation of histones H3 and H4 in the FLI1 gene promoter |
↓ |
FLI1 suppression; increased collagen synthesis |
Human skin, normal and dcSSc fibroblasts |
[57] |
| HDAC-1 and 6 |
↑ |
FLI1 suppression |
Human skin, normal and dcSSc fibroblasts |
[57] |
| MBD-1 and 2, MeCP2 |
↑ |
DNA methylation |
Human skin, normal and dcSSc fibroblasts |
[57] |
| Methylation of CpG islands in the FLI1 promoter |
↑ |
FLI1 suppression; increased Collagen synthesis |
Human skin, normal and dcSSc fibroblasts |
[57] |
| DNMT1 |
↑ |
FLI1 suppression |
Human skin, normal and dcSSc fibroblasts |
[33,57] |
| COL23A1, COL4A2 methylation |
↓ |
Increased collagen synthesis |
Human dcSSc and lcSSc dermal fibroblasts |
[58] |
| ITGA9 methylation |
↓ |
TGF-β upregulation |
Human dcSSc and lcSSc dermal fibroblasts |
[58] |
| ADAM12 methylation |
↓ |
TGF-β upregulation |
Human dcSSc and lcSSc dermal fibroblasts |
[58] |
| miRNA-26a |
↑ |
FLI1 suppression |
Primary SSc skin fibroblasts |
[31] |
| miRNA-21 |
↑ |
TGF-β upregulation;
collagen synthesis |
Human dcSSc and TGF-β treated normal fibroblasts |
[59] |
| miRNA-29a |
↓ |
TGF-β upregulation;
collagen synthesis |
Human dcSSc and TGF-β treated normal fibroblasts |
[59] |