1. Introduction
The first circRNA molecule that is covalently closed was discovered by Sanger et al., (1976) [1] in plant viroids; since then, some studies associated with circRNAs have been published. However, only in 2010 with advances in RNA sequencing technology did several studies emerge linking circRNAs to diseases such as cardiovascular diseases (CVDs) and cancer [2,3,4,5,6,7,8,9].
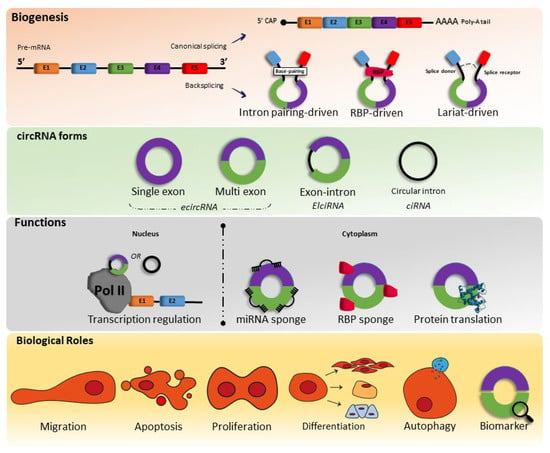
CircRNAs are formed through pre-mRNA backsplicing; during backsplicing, a downstream 5′ splice site is linked to an upstream 3′ splice site in a reverse orientation, thus resulting in a circular RNA molecule with a 3′,5′ phosphodiester bond at the backsplicing junction site [
10]. This process can occur both at the co- and posttranscriptional levels [
10,
11,
12]. However, the circular RNA molecule can be formed in several ways, and until recent studies, are divided into five categories: exonic circular RNA (EcircRNA), where most are located in the cytoplasm, intronic circular RNA (ciRNA), exon–intron circular RNA (EIcircRNA), where the latter two are mostly located nucleus fusion circRNAs (f-circRNAs), and read-through circRNAs (rt-circRNAs) [
10,
11] (
Figure 1).
Figure 1. Biogenesis, forms, functions, and biological roles of circRNAs. miRNA—microRNAs; RBP—RNA-binding proteins.
Therefore, as circRNAs are generated from pre-mRNA backsplicing, as mentioned above, the literature shows that there are three types of models of biogenesis, including intron-pairing-triggered circularization, RNA binding protein (RBP)-driven circularization, and lariat-triggered circularization.
2. circRNAs in Arterial Hypertension
Arterial hypertension (AH) is characterized by a chronic and abnormal rise in blood pressure, with a multifactorial etiology involving genetic, environmental, and social determinants, and is the main contributor to the global burden of CVDs [
23].
Liu et al., [
24] found 485 differentially expressed circRNAs in aortic vascular tissue of spontaneously hypertensive rats (SHR) when compared to Wistar Kyoto (WKT) rats. Three circRNA-miRNA-mRNA axes were predicted and later confirmed in SHR aortas, regulating the NOTCH1, FOXO3, and STAT3 genes, all of which are involved in vascular diseases. The researchers highlighted that some of these differentially expressed circRNAs found in rats were highly similar to homologous sequences in human circRNAs. In another study, researchers used a model of angiotensin-II-induced vascular smooth muscle cell senescence and vascular samples from human hypertensive patients to demonstrate that the overexpression of circACTA2 decreased CDK4 mRNA stability and protein expression, leading to cell senescence [
25]. Endothelial dysfunction, crucial for the development of arterial hypertension, is closely associated with several ncRNAs, especially lncRNAs, circRNAs, and miRNAs [
26]. CircACTA2 may also be involved in vascular function and remodeling by regulating smooth muscle alpha actin (αSMA) expression [
27,
28].
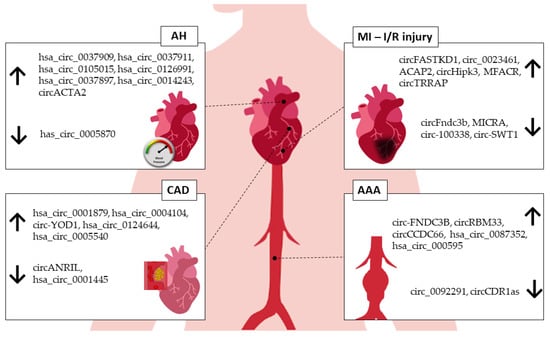
To date, the human circRNAs_0037911, _0126991, and _0005870 seem to be the best candidates as hypertension biomarkers. CircRNA_0037911 and _0126991 were found to be highly regulated in the blood of hypertensive patients, while circRNA_0005870 was downregulated. Taken together, these circRNAs seem to be involved in vascular endothelial dysfunction and, consequently, the development of arterial hypertension.
3. circRNAs in Myocardial Infarction
Myocardial infarction (MI) is a highly prevalent CVD with substantial global morbidity and mortality rates [
37], making MI one of the main focuses of circRNA-related studies, with a large number of described circRNAs [
38]. Ischemia/reperfusion (I/R) injury is one methodology to study the mechanisms that lead to tissue injury in this disease, and we have had a huge increase in the understanding of how ncRNAs are involved in several underlying pathological mechanisms in IR injury, acting as biomarkers or as therapeutic strategies [
39,
40,
41], especially those related to myocardial inflammation, apoptosis, angiogenesis, fibrosis, and oxidative damage.
With a known key role in fibrotic and tumoral diseases [
42], circHIPK3, which is highly expressed in several human tissues, seems to be involved in even more pathophysiological processes. Si et al., [
43] showed the cardiac regeneration effect of circHIPK3 by modulating cardiomyogenesis and angiogenesis after MI. When overexpressed, circHIPK3 increased the proliferation, migration, and tube formation of coronary vessel endothelial cells, attenuated cardiac dysfunction, and decreased the MI-related fibrotic area. Researchers conclude that circHIPK3 may be an ideal candidate as a therapeutic target to improve prognosis after MI. Another study also found that circHIPK3 regulates post-MI cardiac angiogenesis, but in cardiomyocyte-derived exosomes [
44].
Myocardial-infarction-associated circular RNA (MICRA), from the ZNF604 locus, remains the main circRNA biomarker associated with MI. Salgado-Somoza et al., [
45] used the blood levels of MICRA to stratify patients into groups depending on how preserved their left ventricular ejection fraction (LVEF) was four months after MI. MICRA was able to improve the risk classification and was proposed as a biomarker in the prognostication strategy. In another study, Vausort et al., [
46] enrolled infarcted patients from two independent cohorts and found that MI patients had lower MICRA expression levels than healthy subjects. They also indicated MICRA as a strong predictor of left ventricle dysfunction.
4. circRNAs in Coronary Artery Disease
Additionally, known as coronary heart disease or even ischemic heart disease, coronary artery disease (CAD) is characterized by atherosclerotic occlusions of the coronary arteries, possibly leading to myocardial infarction and sudden death, and it is the primary cause of death worldwide [
77,
78]. Conditions such as chronic inflammation and endothelial injuries are involved in the complex etiology of CAD, and some circRNAs have been linked with the development and progression of this disease [
79].
In a mouse model study, Gong et al., [
80] reported the role of circEsyt2 in vascular remodeling by enhancing cell proliferation and migration while inhibited apoptosis and differentiation in vascular smooth muscle cells (VSMC). The experimental findings were in accordance with the higher circEsyt2 expression found in severe-CAD patients when compared to mild-CAD patients, suggesting a proatherosclerotic role of this circRNA. Inversely, circANRIL was found to induce apoptosis and inhibit proliferation, conferring atheroprotection [
81]. The most interesting finding here is that both linear and circular ANRIL were differentially expressed in CAD patients vs. controls, but linear ANRIL showed an atheros progressive function.
Several circRNAs have been proposed as CAD biomarkers thus far. In CAD vs. non-CAD subjects, the combined expression of circRNAs hsa_circ_0001879 and hsa_circ_0004104 showed equal diagnostic value compared to Holter monitoring, treadmill exercise tests, and coronary computed tomography angiography [
82]. hsa_circ_0004104 may also be involved in CAD pathogenesis. hsa_circ_0124644 is also a promising CAD biomarker. In a cohort study, hsa_circ_0124644 was upregulated (2.2-fold change) in the CAD group (n = 137) vs. control group (n = 115). In the CAD vs. control scenario, hsa_circ_0124644 expression had a sensitivity and specificity of 0.86 and 0.62, respectively. The subjects from the CAD group were then classified into two different groups according to disease severity, and hsa_circ_0124644 retained its diagnostic value [
83].
5. circRNAs in Abdominal Aortic Aneurysm
Although there is a lack of consensus, abdominal aortic aneurysm (AAA) could be defined as a greater than 1.5-fold increase in a localized region of the abdominal aorta diameter, with a slow expansion until its rupture [
87]. It is estimated that AAA causes 150,000–200,000 deaths per year worldwide, with higher prevalence in the male, smoker, and older populations [
88,
89]. Rupture is the main AAA complication, with only ~20% chance of survival [
90]; however, cardiovascular events are the most common cause of death in these patients, with increased cardiovascular death risk by approximately 3% per year after AAA diagnosis [
91].
Receiving far less attention than other CVDs, probably due to the difficulty of diagnosis, AAA lacks accurate and reliable detection, risk stratification, and rupture prediction tools [
92], while emerging evidence supports the potential use of molecular biology to act as biomarkers or therapeutic approach in this condition [
93].
Zhou et al., [
94] screened the circRNA expression profile in aortas from four AAA patients. RNA high-throughput sequencing detected 13,295 circRNAs, with 411 differentially expressed (145 upregulated and 266 downregulated) compared to control. The relative downregulated expression of hsa_circ_0005360 in these aortic tissues, confirmed by qRT-PCR, reveals the potential role of this circRNA in AAA pathogenesis since it is an alternatively transcript of AAA-associated LDLR gene [
95]. In addition, circRNA/miRNA interaction analysis predicted one binding site between hsa_circ_0005360 and miR-181b, with miRNA associated with vascular inflammation, atherosclerosis, and aneurysms [
96,
97]. This same expression profile study was added to an integrated analysis [
98] compiling four gene expression datasets, including 23 normal artery tissues and 97 AAA samples, and using different prediction tools (e.g., Gene Ontology, Kyoto Encyclopedia of Genes and Genomes analysis, Targetscan, and CircInteractome). The majority of differently expressed genes (DEGs) (263 upregulated and 177 downregulated) were associated with extracellular matrix, tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β). Bioinformatic analysis, used to build an interaction network between circRNAs, miRNAs, and mRNAs, revealed that the circ_0005073 may modulate several miRNAs affecting the expression of important DEGs in AAA, such as CCL2 (macrophage infiltration), SPP1 (proinflammatory), and UBA52 (protein degradation).
AAA has a complex and multifactorial pathogenic process. Although the literature addressing circRNAs specifically in AAA is still scarce (
Figure 2), the current evidence strongly supports the role of circRNAs in cellular processes that could be associated with the development of AAA, such as the modulation of endothelial cells, macrophages, and VSMCs [
99].
Figure 2. circRNAs associated with human CVDs. AH—arterial hypertension, MI—myocardial infarction, I/R—ischemia/reperfusion injury, CAD—coronary artery disease, AAA—abdominal aortic aneurysm. ↑—upregulated, ↓—downregulated.
6. Exercise-Related circRNAs in the Cardiovascular System
Previous studies reviewed the literature focusing on how CVS-related ncRNA expression responds to physical exercise. The conclusion is quite similar between them: although there is great interest in this topic, we lack studies aiming to describe these underlying molecular mechanisms. Aerobic exercise improved cardiac function and decreased cardiac apoptosis and fibrosis by modulating the expression of a few long noncoding RNAs (lncRNAs): growth arrest specific 5 (GAS5), myocardial-infarction-association transcript (MIAT), metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), and H19 (encoded by the H19 gene) [
111]. Targeting another class of ncRNAs, Wang et al., [
112] described exercised-regulated miRNAs that benefit the heart by promoting protective effects in myocardial fibrosis (miR-29a and miR-29c), ischemia-reperfusion injury (miR-17-3p and miR-222), myocardial infarction (miR-1, miR-214), and heart failure (miR-21-5p, miR-132-3p, miR-208b-3p, miR-212-3p, and miR-335). The majority of studies focus on running and swimming exercise.
With regard to physical-exercise-related circular RNA expression, the evidence is even scarcer. Guo et al., [
113] compared the skeletal muscle circRNA profile in sedentary young versus aged mice and aged mice under aerobic training and identified circBBS9 as a potential biomarker of aging-associated muscle dysfunction regulated by exercise. Niu et al., [
114] found reduced expression of circRIMS2 and brain-derived neurotrophic factor (BDNF) in the serum of patients with vascular cognitive impairment (VCI) and experimentally showed that aerobic exercise improved cognitive function by inhibiting neuronal apoptosis through the circRIMS2/miR-186/BDNF axis. In another study, aerobic exercise combined with glucosamine therapy improved knee osteoarthritis in a rabbit model by enhancing the expression of CircUNK and, consequently, regulating cartilage-differentiation- and apoptosis-related genes. Meinecke et al., [
115] explored the circRNA expression profile in the plasma of marathon runners and proposed circMBOAT2 as a promising biomarker for cardiopulmonary adaptation.
Recently, Zhu et al., [
116] showed the role of the circRNA Circ-Ddx60 in heart failure using physical exercise as preventive treatment. The rationale of this study is based on the report in which physical exercise provided an antihypertrophic memory to myocardial muscle even after the regression of exercise-induced physiological hypertrophy [
117]. In this study, the term exercise hypertrophic preconditioning (EHP) was applied, as preventive intervention, and was followed by a transverse aortic constriction (TAC) or sham surgery. The EHP group showed an increased expression of Circ-Ddx60, and its silencing attenuated the antihypertrophic effect of exercise, worsening heart failure in animals that underwent TAC.
To the extent of our knowledge, this is the first research article to explore the potential of exercise-related circRNA in a cardiovascular disease, providing interesting insights in this vast research field.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24032125