Cistus ladanifer (rockrose) is a widespread shrub species in the Mediterranean region well known due to its production of labdanum gum, especially in the hot season. Its leaves and branches can be subjected to different extraction and distillation processes to produce various types of extracts. The natural extracts of C. ladanifer have several applications, especially in the perfumery and cosmetics sector. C. ladanifer extracts, in addition to presenting interesting odoriferous properties, are also known for their bioactive properties, such as antioxidant and antimicrobial. The use of this species in animal feed or phytostabilisation of mining areas has also been successfully applied. Furthermore, the lignin and polysaccharides that are the major fractions from Cistus residues can be relevant sources of high-value products in a biorefinery framework.
- added-value products
- bioeconomy
- biofuels
- essential oils
- integrated upgrade
- rockrose
1. Introduction

2. Traditional Products from Rockrose
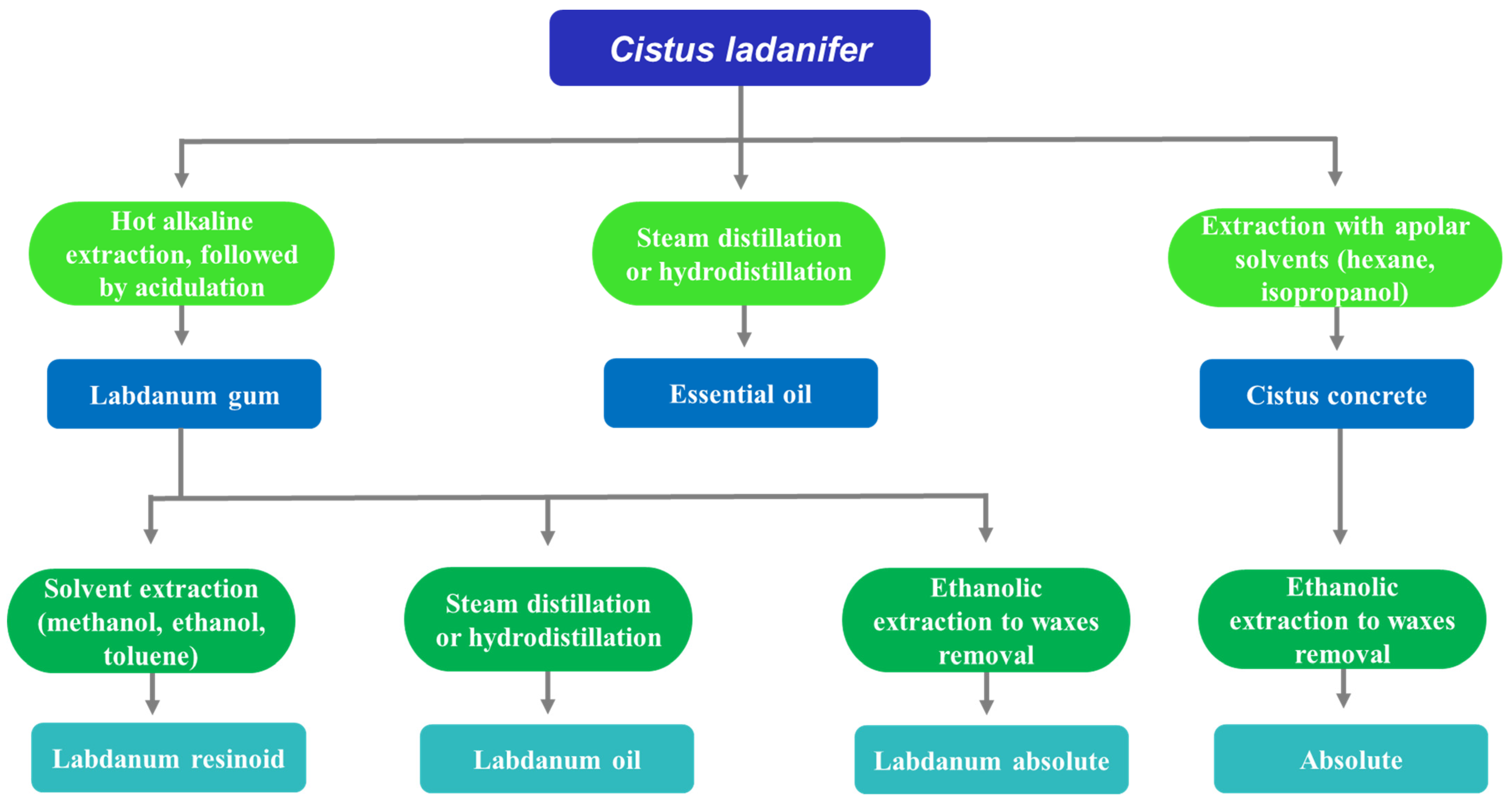
2.1. Aromatic Extracts
|
Component |
Centre Interior of Portugal a |
Central Spain a |
Corsica b |
Eastern Morocco a |
|---|---|---|---|---|
|
Monoterpene hydrocarbons |
||||
|
Tricyclene |
- |
- |
- |
2.7 |
|
α-Pinene |
2.1 |
4.70 |
39 |
4.2 |
|
Camphene |
0.3 |
0.64 |
2.1 |
15.5 |
|
Pinocarvone |
1.1 |
- |
0.9 |
- |
|
Limonene |
- |
0.37 |
1.7 |
- |
|
γ-Terpinene |
- |
0.10 |
0.4 |
3.8 |
|
α-Terpinene |
- |
- |
0.1 |
1.8 |
|
p-cymenene |
- |
1.17 |
1.7 |
2.3 |
|
Oxygenated monoterpenes |
||||
|
Bornyl acetate |
1.6 |
7.03 |
3.1 |
|
|
Terpinen-4-ol |
1.0 |
6.37 |
1.1 |
6.3 |
|
α-Terpineol |
- |
2.20 |
- |
1.2 |
|
trans-pinocarveol |
2.1 |
20.00 |
1.9 |
|
|
Borneol |
0.7 |
- |
0.8 |
11.1 |
|
Myrtenal |
0.7 |
2.26 |
0.5 |
- |
|
cis-Pinocamphone |
- |
3.84 |
- |
- |
|
2 (10)-Pinen-3-one |
- |
5.05 |
- |
- |
|
Verbonene |
- |
0.85 |
0.3 |
0.8 |
|
Camphor |
- |
0.86 |
- |
1.5 |
|
p-Mentha-1,5-dien-8-ol |
- |
4.78 |
- |
- |
|
Sesquiterpene hydrocarbons |
||||
|
Viridiflorene |
1.3 |
0.41 |
- |
- |
|
C15H26O sesquiterpene alcohol |
6.0 |
- |
- |
- |
|
Cyclosativene |
- |
0.70 |
0.7 |
0.6 |
|
Aromadendrene |
- |
1.77 |
- |
- |
|
Allo-aromadendrene |
0.8 |
1.9 |
- |
|
|
α-Copaene |
- |
0.62 |
0.8 |
- |
|
α-Cubebene |
- |
- |
- |
2.2 |
|
δ-cadinene |
1.0 |
- |
0.8 |
6.4 |
|
Oxygenated sesquiterpenes |
||||
|
Viridiflorol |
17.4 |
13.59 |
11.8 |
2.8 |
|
Spathulenol |
0.8 |
0.53 |
0.5 |
- |
|
Globulol |
5.0 |
- |
0.3 |
- |
|
Ledol |
- |
4.36 |
3.3 |
- |
|
Caryophyllene oxide |
1.8 |
- |
- |
- |
|
Palustrol |
- |
0.50 |
- |
- |
|
Others |
||||
|
2,2,6-trimethylcyclohexanone |
2.8 |
- |
0.9 |
7.3 |
|
Phthalates |
||||
|
Diethyl phthalate |
- |
- |
- |
2.9 |
|
Bis (2-ethylhexyl) phthalate |
- |
- |
- |
0.2 |
|
Material used for hydrodistillation |
dry leaves and small branches |
fresh leaves |
leaves and stems |
dry leaves |
a components identified using gas chromatography (GC) and/or gas chromatography/mass spectrometry (GC/MS); b components identified by C-NMR spectroscopy and GC.
2.2. Extractives
|
Compound |
Chemical Group |
Part of the Plant |
References |
|
Volatile compounds |
|
||
|
α-Pinene |
Monoterpene hydrocarbons |
Aerial part; shoots |
|
|
Camphene |
Aerial part; shoots |
||
|
Pinocarvone |
Aerial part; shoots |
||
|
Limonene |
Aerial part |
[45] |
|
|
α-Phellandrene |
Leaves |
[47] |
|
|
γ-Terpinene |
Aerial part |
[45] |
|
|
α -Thujene |
Aerial part |
[45] |
|
|
p-cymene |
Aerial part |
[45] |
|
|
Bornyl acetate |
Oxygenated monoterpenes |
Aerial part; shoots |
|
|
Terpinen-4-ol |
Aerial part; shoots |
||
|
α-Campholenal |
Aerial part |
[45] |
|
|
trans-pinocarveol |
Aerial part |
[45] |
|
|
Borneol |
Leaves; aerial part |
[45] |
|
|
Myrtenal |
Aerial part |
[45] |
|
|
(cis)-Verbenol |
Leaves; shoots |
||
|
Verbonene |
Leaves; aerial part; shoots |
||
|
Camphor |
Leaves; shoots |
||
|
Viridiflorol |
Shoots |
[46] |
|
|
Globulol |
Oxygenated sesquiterpenes |
Shoots |
[46] |
|
Ledol |
Leaves |
[47] |
|
|
Caryophyllene oxide |
Shoots |
[46] |
|
|
Eugenol |
Phenylpropene |
Leaves |
[47] |
|
Benzenepropanoic acid |
Phenylpropanoid |
Shoots |
[46] |
|
2-Phenylethanol |
Alcohol |
Leaves; aerial part |
|
|
Acetophenone |
Aromatic ketone |
Leaves |
[47] |
|
Thuja-2,4(10)-diene |
Others |
Aerial part |
[45] |
|
Rhododendrol |
Shoots |
[46] |
|
|
2,2,6-trimethylcyclohexanone |
Leaves; aerial part; shoots |
||
|
Soluble compounds (phenolics) |
|
||
|
Apigenin |
Flavonoids |
Leaves; aerial part; whole plant |
|
|
Apigenin-6-C-glucose-8-C-glucose |
Leaves |
[51] |
|
|
Apigenin methylether |
Whole plant |
[48] |
|
|
Kaempferol dimethylether |
Aerial part; leaves; whole plant |
||
|
Kaempferol diglycoside |
Whole plant |
[48] |
|
|
4´(o)methyl-apigenin |
Leaves |
[49] |
|
|
7(o)methyl-apigenin |
Leaves |
[49] |
|
|
3-methyl-kaempferol |
Leaves |
[49] |
|
|
4´-dimethyl-kaempferol |
Leaves |
[49] |
|
|
3,7-dimethyl-kaempferol |
Leaves |
[49] |
|
|
3,7,4´-trimethyl-kaempferol |
Leaves |
[49] |
|
|
Kaempferol methylether |
Aerial part; leaves |
||
|
Quercetin-O-hexoside-Ohexoside |
leaves |
[51] |
|
|
Epigallocatechin |
Aerial part; leaves |
||
|
Gallic acid |
Phenolic acids and |
Aerial part |
[50] |
|
Glucogallin (isomer) |
Aerial part |
[50] |
|
|
Gentisoyl glucoside |
Aerial part; whole plant |
||
|
Digaloil-β-D-glucopiranose |
Aerial part |
[50] |
|
|
Galloyl glucose |
Leaves |
[51] |
|
|
Mirciaphenone B |
Aerial part |
[50] |
|
|
Punicalagin isomer 1 |
Ellagic acid and |
Aerial part, leaves |
|
|
Punicalagin isomer 2 |
Aerial part, leaves |
||
|
Punicalagin gallate 1 |
Leaves |
[51] |
|
|
Punicalagin gallate 2 |
Leaves |
[51] |
|
|
Punicalin |
Aerial part; whole plant |
||
|
Cornusiin |
Aerial part |
[50] |
|
|
Ellagic acid-7-xyloside |
Aerial part |
[50] |
|
|
Ellagic acid |
Aerial part |
[50] |
|
|
Ducheside A |
Aerial part |
[50] |
|
|
Shikimic acid |
Others |
Aerial part Whole plant |
|
|
Quinic acid |
Aerial part Whole plant |
||
|
Hexahydroxydiphenoyl-D-glucose (isomer) |
Aerial part |
[50] |
|
|
Phenethyl-β-primeveroside |
Aerial part |
[50] |
|
3. Bioactivity
|
Plant Part |
Type of Extract |
Biological Activities |
References |
|---|---|---|---|
|
Fresh leaves from flowering stems |
Methanol/water
|
Antifungal |
[67] |
|
Leaves |
Aqueous |
Autotoxicity |
[12] |
|
Wood/stalks, bark, and leaves |
Ethanol and |
Antioxidant |
[68] |
|
Leaves |
Aqueous |
Allelopathic |
[58] |
|
Aerial parts |
Aqueous |
Antihypertensive |
[69] |
|
Whole plant |
Hydroalcoholic and spray-dried/spray-dried aqueous extract |
Antibacterial |
[4] |
|
Leaves |
Aqueous |
Antioxidant Antimicrobial, Cytotoxic activity against human cancer cells |
[59] |
|
Leaves |
Flavonoids |
Allelopathic |
[70] |
|
Shoots |
Water-soluble and volatile compounds |
Phytotoxic |
[71] |
|
Leaves and small branches Whole plant |
Essential oil Labdanum |
Antitungical, Antibacterial |
[28] |
|
Aerial parts |
Essential oil |
Herbicidal activity |
[39] |
|
Fruits, stems, flowers, and leaves |
Essential oil water, ethanol, ethanol: water (50:50), methanol, methanol: water (50:50), acetonitrile |
Antioxidant |
[41] |
|
Aerial parts |
Hydrolates volatiles Essential oil |
Antioxidant Anti-inflammatory Antimicrobial |
[72] |
4. Novel and Potential Applications
Other potential applications have been suggested for C. ladanifer besides the traditional use of extracts in their already well established application in the perfumery industry as a fixative of perfumes. Some of the new directions regarding the valorization of rockrose include phytoremediation of soils contaminated by heavy metals, use of the plant for feed with nutritional benefits for animal health, and use as a lignocellulosic raw material for added value products, namely the production of bioethanol.
4.1. Phytoremediation
4.2. Animal Feed
4.3. Added-Value Products from Lignocellulosic Material
Recently, the researchers presented a set of works that suggest an integrated upgrading strategy for C. ladanifer distillery biomass residues obtained after essential oil steam distillation. The strategy started with the removal of extractives (40% of the dry biomass), producing an extract rich in phenolic compounds (mainly gallic acid and flavonoids) with antioxidant properties [89][90]. The remaining lignocellulosic material, containing mainly polysaccharides (51%) and lignin (33%), was subjected to selective fractionation processes for sequential recovery of hemicelluloses by an autohydrolysis process [89] and lignin by organosolv and alkaline processes [93], producing a solid enriched in cellulose that had increased enzymatic digestibility (approximately four times higher than the initial feedstock) [94].
Optimization of the autohydrolysis process enabled to obtain XOS with potential prebiotic activity, in a maximum concentration of 16 g/L, corresponding to a yield of 10.2 g/100 g extracted feedstock [89]. The alkaline and organosolv delignification processes affected the monomeric composition of the residual lignin, with a decrease in the S/G ratio (quantified by analytic pyrolysis, Py-GC/MS) and solubilization and recovery of several phenolic compounds with high added value, namely vanillic acid, p-coumaric acid, and epicatechin [93]. The alkaline treatments lead to higher delignification (87%) and subsequent higher cellulose enzymatic saccharification yields (79%) [93]. Glucan-rich solids and pentoses-rich hemicellulosic hydrolysates were used, separately or together, for the selective production of the D-lactic acid isomer (D-LA) by the recombinant strain E. coli JU15 in different fermentation modes: simultaneous saccharification and co-fermentation (SSCF), SHF, and SSF. In this study, high lactic acid yields, in the range of 92–99 g D-LA/100 g sugars were achieved [95].
5. Future Perspectives
The use of C. ladanifer, not only as a source of essential oils or labdanum gum but also as a feedstock to obtain other products, namely from its industrial residues, can be strategic in the expansion of essential oil distilleries and consequently in the development of rural areas where endogenous biomass potential is still poorly explored, promoting the bioeconomy and circular economy models.
The possible integration of processes and products for the valorization of C. ladanifer in a biorefinery framework is shown in Figure 3 with full resource use and exploitation to obtain added-value products or novel applications, conveying the biorefinery goals, i.e., integral and sustainable use of biomass for concomitant production of biofuels, energy, materials, and chemicals, preferably with added value [85].
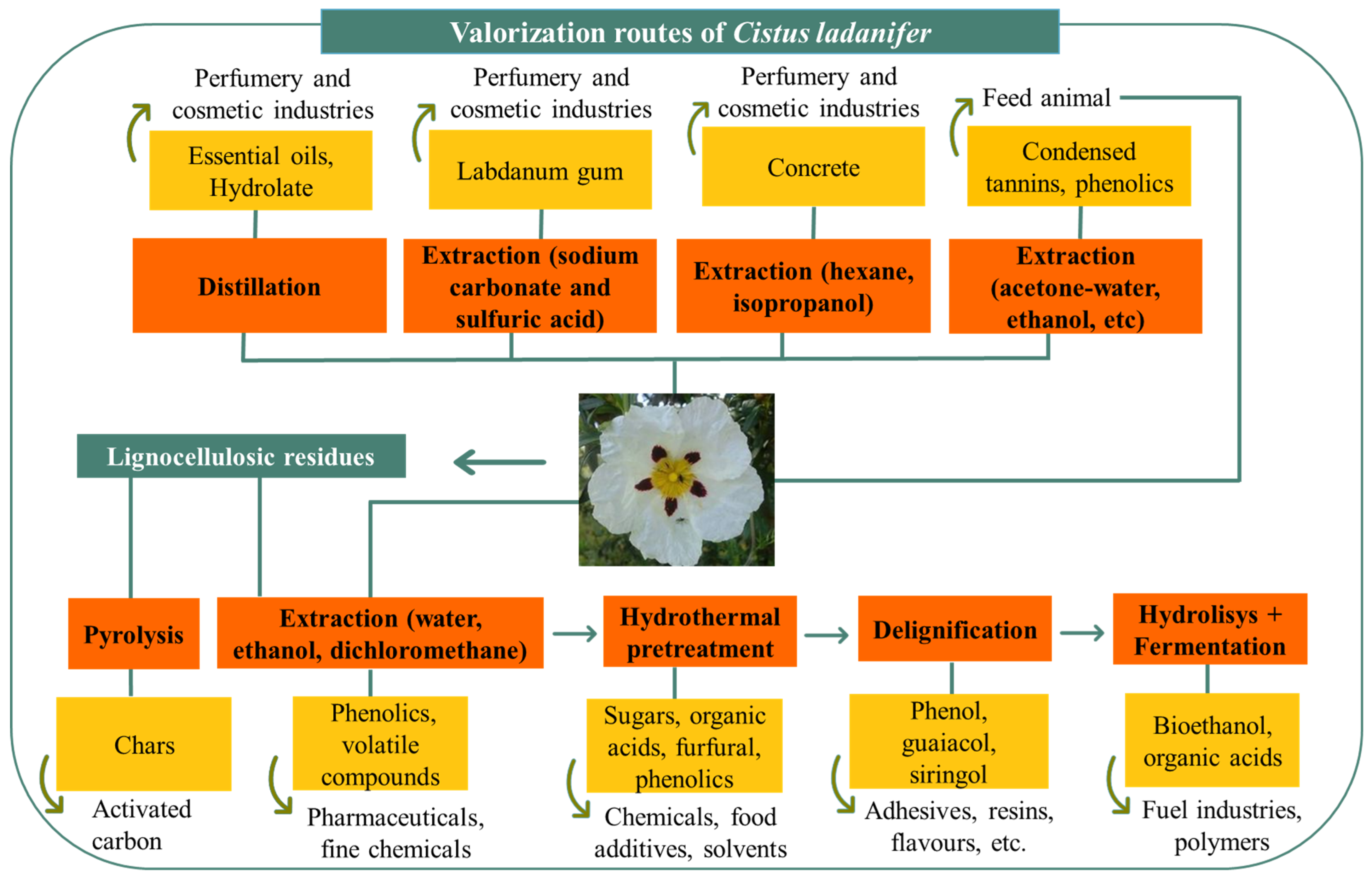
Figure 3. Potential path for use of Cistus ladanifer with an integrated valorization in a biorefinery framework for obtaining different added-value products.
Valorization of C. ladanifer may contribute to better use of an underexploited endogenous resource while promoting residue management, reducing pressure on the environment, and promoting sustainable development through the creation of new products and the generation of new jobs. Nevertheless, to shift biorefineries into industrial reality, the development of a distribution map highlighting local potential availability and aspects related to logistics of transport must be taken into consideration to build a complete sustainability analysis. Additionally, a comparative techno-economic analysis of the different processes, namely with the help of modeling tools, as well as a life cycle assessment in terms of environmental, social, and economic sustainability should also be carried out before selecting biomass commercial valorization pathways.
This entry is adapted from the peer-reviewed paper 10.3390/en16010391
References
- Heywood, V.H. (Ed.) Las Plantas Con Flores; Reverté: Barcelona, Spain, 1985.
- Weyerstahl, P.; Marschall, H.; Weirauch, M.; Thefeld, K.; Surburg, H. Constituents of Commercial Labdanum Oil. Flavour Fragr. J. 1998, 13, 295–318.
- Ferreira, S.; Duarte, A.P.; Queiroz, J.A.; Domingues, F.C. Experimental Design for Enzymatic Hydrolysis of Cistus ladanifer. J. Biotechnol. 2008, 136, S274–S275.
- Tomas-Menor, L.; Morales-Soto, A.; Barrajon-Catalan, E.; Roldan-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various Spanish Cistus Species. Food Chem. Toxicol. 2013, 55, 313–322.
- Talavera, S.; Gibbs, P.E.; Herrera, J. Reproductive Biology of Cistus ladanifer (Cistaceae). Plant Syst. Evol. 1993, 186, 123–134.
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile Profile of Spanish Cistus Plants as Sources of Antimicrobials for Industrial Applications. Ind. Crops Prod. 2015, 74, 425–433.
- Guzmán, B.; Vargas, P. Long-Distance Colonization of the Western Mediterranean by Cistus ladanifer (Cistaceae) despite the Absence of Special Dispersal Mechanisms. J. Biogeogr. 2009, 36, 954–968.
- Ferreira, M.R.; Almeida, A.M.; Quintela-Sabarís, C.; Roque, N.; Fernandez, P.; Ribeiro, M.M. The Role of Littoral Cliffs in the Niche Delimitation on a Microendemic Plant Facing Climate Change. PLoS ONE 2021, 16, e0258976.
- Carlier, J.; LeitÃo, J.; Fonseca, F. Population Genetic Structure of Cistus ladanifer L. (Cistaceae) and Genetic Differentiation from Co-Occurring Cistus Species. Plant Species Biol. 2008, 23, 141–151.
- Demoly, J.-P.; Montserrat, P. Cistus; Castroviejo, S., Aedo, C., Laínz, M., Muñoz Garmendia, F., Nieto Feliner, G., Paiva, J., Benedí, C., Eds.; Flora iber; CSIC: Madrid, Spain, 1993.
- Borges, A.E.L. Contribuição Para o Estudo Da Anatomia Da Folha e Caule de Cistus ladanifer L. In Proceedings of the I Jornadas Ibericas de Plantas Medicinales, Aromaticas y de Aceites Esenciales, Madrid, Spain, 12–14 July 1989; pp. 119–128.
- Alías, J.C.; Sosa, T.; Escudero, J.C.; Chaves, N. Autotoxicity Against Germination and Seedling Emergence in Cistus ladanifer L. Plant Soil 2006, 282, 327–332.
- Brickell, C. Gardeners’ Encyclopedia of Plants and Flowers; Dorling Kindersley: London, UK, 1989.
- Raimundo, J.R.; Frazão, D.F.; Domingues, J.L.; Quintela-Sabarís, C.; Dentinho, T.P.; Anjos, O.; Alves, M.; Delgado, F. Neglected Mediterranean Plant Species Are Valuable Resources: The Example of Cistus ladanifer. Planta 2018, 248, 1351–1364.
- Iriondo, J.M.; Moreno, C.; Pérez, C. Micropropagation of Six Rockrose (Cistus) Species. Hortscience 1995, 30, 1080–1081.
- Kidd, P.S.; Díez, J.; Monterroso Martínez, C. Tolerance and Bioaccumulation of Heavy Metals in Five Populations of Cistus ladanifer L. Subsp. Ladanifer. Plant Soil 2004, 258, 189–205.
- Rossini-Oliva, S.; Mingorance, M.D.; Monaci, F.; Valdés, B. Ecophysiological Indicators of Native Cistus ladanifer L. at Riotinto Mine Tailings (SW Spain) for Assessing Its Potential Use for Rehabilitation. Ecol. Eng. 2016, 91, 93–100.
- Simões, M.P.; Madeira, M.; Gazarini, L. The Role of Phenology, Growth and Nutrient Retention during Leaf Fall in the Competitive Potential of Two Species of Mediterranean Shrubs in the Context of Global Climate Changes. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 578–589.
- Santos, E.; Abreu, M.M.; Nabais, C.; Saraiva, J. Antioxidant Enzymes Activity of Cistus ladanifer L. from Areas Non-Contaminated in Trace Elements. Rev. Ciênc. Agrár. 2011, 34, 32–43.
- Pang, J.; Chan, G.S.Y.; Zhang, J.; Liang, J.; Wong, M.H. Physiological Aspects of Vetiver Grass for Rehabilitation in Abandoned Metalliferous Mine Wastes. Chemosphere 2003, 52, 1559–1570.
- Calvo, L.; Tárrega, R.; Luis, E.; Valbuena, L.; Marcos, E. Recovery after Experimental Cutting and Burning in Three Shrub Communities with Different Dominant Species. Plant Ecol. 2005, 180, 175–185.
- Tárrega, R.; Luis-Calabuig, E.; Valbuena, L. Eleven Years of Recovery Dynamic after Experimental Burning and Cutting in Two Cistus Communities. Acta Oecol. 2001, 22, 277–283.
- Pérez-García, F. Germination of Cistus ladanifer in Relation to Parent Material. Plant Ecol. 1997, 133, 57–62.
- Nuñez, E. Ecología Del Jaral de Cistus ladanifer L.; Universidad de Extremadura: Badajoz, Spain, 1989.
- Gallego, J.C.A. Influencia de Los Factores Climáticos En La Síntesis y Actividad de Compuestos Fitotóxicos Secretados Por Cistus ladanifer L.; Universidad de Extremadura: Badajoz, Spain, 2006.
- Delgado, J.A.; Serrano, J.M.; López, F.; Acosta, F.J. Seed Size and Seed Germination in the Mediterranean Fire-Prone Shrub Cistus Ladanifer. Plant Ecol. 2007, 197, 269–276.
- Narbona, E.; Guzmán, B.; Arroyo, J.; Vargas, P. Why Are Fruit Traits of Cistus ladanifer (Cistaceae) so Variable: A Multi-Level Study across the Western Mediterranean Region. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 305–315.
- Greche, H.; Mrabet, N.; Zrira, S.; Ismaïli-Alaoui, M.; Benjilali, B.; Boukir, A. The Volatiles of the Leaf Oil of Cistus ladanifer L. Var. Albiflorus and Labdanum Extracts of Moroccan Origin and Their Antimicrobial Activities. J. Essent. Oil Res. 2009, 21, 166–173.
- Gomes, P.B.; Mata, V.G.; Rodrigues, A.E. Characterization of the Portuguese-Grown Cistus ladanifer Essential Oil. J. Essent. Oil Res. 2005, 17, 160–165.
- Teixido, A.L.; Méndez, M.; Valladares, F. Flower Size and Longevity Influence Florivory in the Large-Flowered Shrub Cistus ladanifer. Acta Oecol. 2011, 37, 418–421.
- Hernández-Rodríguez, M.; de-Miguel, S.; Pukkala, T.; Oria-de-Rueda, J.A.; Martín-Pinto, P. Climate-Sensitive Models for Mushroom Yields and Diversity in Cistus ladanifer Scrublands. Agric. For. Meteorol. 2015, 213, 173–182.
- Santos, E.S.; Balseiro-Romero, M.; Abreu, M.M.; Macías, F. Bioextracts of Cistus ladanifer L. Growing in São Domingos Mine as Source of Valuable Compounds. J. Geochem. Explor. 2016, 174, 84–90.
- Pérez, P.; Saúl, L.; Ciria, M.P. Distribución Geográfica, Caracterización Ecológica y Evaluación de Cistus laurifolius y Cistus ladanifer. Estudios Sobre El Matorral Como Recurso Energético. Agroenerg. Biomassa Vida Rural 2011, 331, 66–70.
- Lourenço, K.R.; Costa, M.C.; Palma, M.L. Possibilidades Terapêuticas da Esteva (Cistus ladanifer L.). Rev. Fitoter. 2015, 15, 115–126.
- Morgado, J.M.; Tapias, R.; Alesso, P. Producción de Goma Bruta de Jara (Cistus ladanifer L.) En El Suroeste de La Península Ibérica. In Proceedings of the Actas 4 Congreso Forestal Español, Zaragoza, Spain, 26–30 September 2005.
- Barrajón-Catalán, E.; Tomás-Menor, L.; Morales-Soto, A.; Bruñá, N.M.; López, D.S.; Segura-Carretero, A.; Micol, V. Rockroses (Cistus sp.) Oils In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124166417.
- Biolandes Cistus Labdanum in Andalusia. Available online: https://www.biolandes.com/catalogue-extraits-naturels/ (accessed on 1 February 2022).
- Viuda-Martos, M.; Sendra, E.; Alvarez, J.A.P.; Fernández-López, J.; Amensour, M.; Abrini, J. Identification of Flavonoid Content and Chemical Composition of the Essential Oils of Moroccan Herbs: Myrtle (Myrtus communis L.), Rockrose (Cistus ladanifer L.) and Montpellier Cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 1–9.
- Verdeguer, M.; Blazquez, M.A.; Boira, H. Chemical Composition and Herbicidal Activity of the Essential Oil from a Cistus ladanifer L. Population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609.
- Mariotti, J.P.; Tomi, F.; Casanova, J.; Costa, J.; Bernardini, A.F. Composition of the Essential Oil of Cistus ladaniferus L. Cultivated in Corsica (France). Flavour Fragr. J. 1997, 12, 147–151.
- Zidane, H.; Elmiz, M.; Aouinti, F.; Tahani, A.; Wathelet, J.; Sindic, M.; Elbachiri1, A. Chemical Composition and Antioxidant Activity of Essential Oil, Various Organic Extracts of Cistus ladanifer and Cistus libanotis Growing in Eastern Morocco. Afr. J. Biotechnol. 2013, 12, 5314–5320.
- Pereira, H.; Graça, J.; Rodrigues, J.C. Wood Chemistry in Relation to Quality. In Wood Quality and Its Biological Basis; Barnett, J.R., Jeronimidis, G., Eds.; Blackwell Publishing: Oxford, UK, 2003; ISBN 1-84127-319-8.
- Rowe, J.W.; Conner, A.H. Extractives in Eastern Hardwoods-A Review; U.S. Department of Agriculture: Madison, WI, USA, 1979.
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and Characterization of Bioactive Compounds from Plant Resources: The Role of Analysis in the Ethnopharmacological Approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228.
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile Profile of Spanish Cistus Plants as Sources of Antimicrobials for Industrial Applications. Ind. Crops Prod. 2015, 74, 425–433.
- Santos, E.S.; Balseiro-Romero, M.; Abreu, M.M.; Macías, F. Bioextracts of Cistus ladanifer L. Growing in São Domingos Mine as Source of Valuable Compounds. J. Geochem. Explor. 2016, 174, 84–90.
- Ramalho, P.S.; de Freitas, V.A.P.; Macedo, A.; Silva, G.; Silva, A.M.S. Volatile Components Of Cistus ladanifer Leaves. Flavour Fragr. J. 1999, 14, 300–302.
- Tomas-Menor, L.; Morales-Soto, A.; Barrajon-Catalan, E.; Roldan-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various Spanish Cistus Species. Food Chem. Toxicol. 2013, 55, 313–322.
- Chaves, N.; Ríos, J.J.; Gutierrez, C.; Escudero, J.C.; Olías, J.M. Analysis of Secreted Flavonoids of Cistus ladanifer L. by High-Performance Liquid Chromatography–Particle Beam Mass Spectrometry. J. Chromatogr. A 1998, 799, 111–115.
- Fernandez-Arroyo, S.; Barrajon-Catalan, E.; Micol, V.; Segura-Carretero, A.; Fernandez-Gutierrez, A. High-Performance Liquid Chromatography with Diode Array Detection Coupled to Electrospray Time-of-Flight and Ion-Trap Tandem Mass Spectrometry to Identify Phenolic Compounds from a Cistus ladanifer Aqueous Extract. Phytochem. Anal. 2010, 21, 307–313.
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C. Antifungal Activity and Detailed Chemical Characterization of Cistus ladanifer Phenolic Extracts. Ind. Crops Prod. 2013, 41, 41–45.
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A Model for Exploring Labdane-Type Diterpenes’ Biosynthesis and a Natural Source of High Value Products with Biological, Aromatic, and Pharmacological Properties. Front. Chem. 2014, 2, 35.
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160.
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. Phenolic Compd. Biol. Act. 2017, 8, 1–24.
- Fernandez-Arroyo, S.; Barrajon-Catalan, E.; Micol, V.; Segura-Carretero, A.; Fernandez-Gutierrez, A. High-Performance Liquid Chromatography with Diode Array Detection Coupled to Electrospray Time-of-Flight and Ion-Trap Tandem Mass Spectrometry to Identify Phenolic Compounds from a Cistus ladanifer Aqueous Extract. Phytochem. Anal. 2010, 21, 307–313.
- Sosa, T.; Alías, J.C.; Escudero, J.C.; Chaves, N. Interpopulational Variation in the Flavonoid Composition of Cistus ladanifer L. Exudate. Biochem. Syst. Ecol. 2005, 33, 353–364.
- Chaves, N.; Ríos, J.J.; Gutierrez, C.; Escudero, J.C.; Olías, J.M. Analysis of Secreted Flavonoids of Cistus ladanifer L. by High-Performance Liquid Chromatography–Particle Beam Mass Spectrometry. J. Chromatogr. A 1998, 799, 111–115.
- Herranz, J.M.; Ferrandis, P.; Copete, M.A.; Duro, E.M.; Zalacaín, A. Effect of Allelopathic Compounds Produced by Cistus ladanifer on Germination of 20 Mediterranean Taxa. Plant Ecol. 2005, 184, 259–272.
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Saura, D.; Guillen, E.; Fernandez-Gutierrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae Aqueous Extracts Containing Ellagitannins Show Antioxidant and Antimicrobial Capacity, and Cytotoxic Activity against Human Cancer Cells. Food Chem. Toxicol. 2010, 48, 2273–2282.
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal Variation of Cistus ladanifer L. Diterpenes. Plants 2012, 1, 6–15.
- Chaves, N.; Escudero, J.C.; Gutierrez-Merino, C. Role of Ecological Variables in the Seasonal Variation of Flavonoid Content of Cistus ladanifer Exudate. J. Chem. Ecol. 1997, 23, 579–603.
- Guerreiro, O.; Dentinho, M.T.P.; Moreira, O.C.; Guerra, A.R.; Ramos, P.A.B.; Bessa, R.J.B.; Duarte, M.F.; Jerónimo, E. Potential of Cistus ladanifer L. (Rockrose) in Small Ruminant Diets–Effect of Season and Plant Age on Chemical Composition, in Vitro Digestibility and Antioxidant Activity. Grass Forage Sci. 2016, 71, 437–447.
- Micco, V.; Aronne, G. Anatomical Features, Monomer Lignin Composition and Accumulation of Phenolics in 1-year-old Branches of the Mediterranean Cistus ladanifer L. Bot. J. Linn. Soc. 2007, 155, 361–371.
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Sousa, M.J.; Morais, J.S.; Ferreira, I.C. Aromatic Plants as a Source of Important Phytochemicals: Vitamins, Sugars and Fatty Acids in Cistus ladanifer, Cupressus lusitanica and Eucalyptus gunnii Leaves. Ind. Crops Prod. 2009, 30, 427–430.
- Guerreiro, O.; Alves, S.P.; Duarte, M.F.; Bessa, R.J.B.; Jerónimo, E. Cistus ladanifer L. Shrub Is Rich in Saturated and Branched Chain Fatty Acids and Their Concentration Increases in the Mediterranean Dry Season. Lipids 2015, 50, 493–501.
- Patra, J.K.; Das, G.; Lee, S.; Kang, S.S.; Shin, H.S. Selected Commercial Plants: A Review of Extraction and Isolation of Bioactive Compounds and Their Pharmacological Market Value. Trends Food Sci. Technol. 2018, 82, 89–109.
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C. Antifungal Activity and Detailed Chemical Characterization of Cistus ladanifer Phenolic Extracts. Ind. Crops Prod. 2013, 41, 41–45.
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive Extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crops Prod. 2009, 30, 165–167.
- Belmokhtar, M.; Bouanani, N.E.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Aziz, M.; Matéo, P.; Fischmeister, R.; Legssyer, A. Antihypertensive and Endothelium-Dependent Vasodilator Effects of Aqueous Extract of Cistus ladaniferus. Biochem. Biophys. Res. Commun. 2009, 389, 145–149.
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant Growth Inhibiting Flavonoids in Exudate of Cistus ladanifer and in Associated Soils. J. Chem. Ecol. 2001, 27, 623–631.
- Dias, L.S.; Moreira, I. Interaction between Water Soluble and Volatile Compounds of Cistus ladanifer L. Chemoecology 2002, 12, 77–82.
- Tavares, C.S.; Martins, A.; Faleiro, M.L.; Miguel, M.G.; Duarte, L.C.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Bioproducts from Forest Biomass: Essential Oils and Hydrolates from Wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind. Crops Prod. 2020, 144, 112034.
- Hooda, V. Phytoremediation of Toxic Metals from Soil and Waste Water. J. Environ. Biol. 2007, 28, 367–376.
- Alvarenga, P.M.; Araújo, M.F.; Silva, J.A.L. Elemental Uptake and Root-Leaves Transfer in Cistus ladanifer L. Growing in a Contaminated Pyrite Mining Area (Aljustrel-Portugal). Water Air Soil Pollut. 2004, 152, 81–96.
- Abreu, M.M.; Santos, E.; Fernandes, E.; Joao, M.; Ferreira, M. Acumulação e Translocação de Elementos Vestigiais Em Cistus ladanifer L. de Áreas Mineiras Da FPI Portuguesa. Rev. Ciênc. Agrár. 2011, 34, 44–56.
- Santos, E.S.; Abreu, M.M.; Nabais, C.; Magalhães, M.C.F. Trace Element Distribution in Soils Developed on Gossan Mine Wastes and Cistus ladanifer L. Tolerance and Bioaccumulation. J. Geochem. Explor. 2012, 123, 45–51.
- Abreu, M.M.; Magalhães, M.C. Phytostabilization of Soils in Mining Areas. Case Studies from Portugal. In Soil Remediation; Aachen, L., Eichmann, P., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2009; pp. 297–344.
- Jerónimo, E.; Alves, S.P.; Dentinho, M.T.; Martins, S.V.; Prates, J.A.; Vasta, V.; Santos-Silva, J.; Bessa, R.J. Effect of Grape Seed Extract, Cistus ladanifer L., and Vegetable Oil Supplementation on Fatty Acid Composition of Abomasal Digesta and Intramuscular Fat of Lambs. J. Agric. Food Chem. 2010, 58, 10710–10721.
- Jerónimo, E.; Alfaia, C.M.; Alves, S.P.; Dentinho, M.T.; Prates, J.A.; Vasta, V.; Santos-Silva, J.; Bessa, R.J. Effect of Dietary Grape Seed Extract and Cistus ladanifer L. in Combination with Vegetable Oil Supplementation on Lamb Meat Quality. Meat Sci. 2012, 92, 841–847.
- Zamora-Lozano, M.; Mata-Moreno, C.; Martínez-Teruel, A.; Gómez-Castro, A.G.; Peinado Lucena, E.; Medina-Blanco, M. Utilización de Cistus ladanifer (L.) En Piensos Para Conejos. Arch. Zootec. 1984, 33, 295–300.
- Dentinho, M.T.P.; Belo, A.T.; Bessa, R.J.B. Digestion, Ruminal Fermentation and Microbial Nitrogen Supply in Sheep Fed Soybean Meal Treated with Cistus ladanifer L. Tannins. Small Rumin. Res. 2014, 119, 57–64.
- Dentinho, M.T.P.; Paulos, K.; Francisco, A.; Belo, A.T.; Jerónimo, E.; Almeida, J.; Bessa, R.J.B.; Santos-Silva, J. Effect of Soybean Meal Treatment with Cistus ladanifer Condensed Tannins in Growth Performance, Carcass and Meat Quality of Lambs. Livest. Sci. 2020, 236, 104021.
- Guerreiro, O.; Alves, S.P.; Soldado, D.; Cachucho, L.; Almeida, J.M.; Francisco, A.; Santos-Silva, J.; Bessa, R.J.B.; Jerónimo, E. Inclusion of the Aerial Part and Condensed Tannin Extract from Cistus ladanifer L. in Lamb Diets–Effects on Growth Performance, Carcass and Meat Quality and Fatty Acid Composition of Intramuscular and Subcutaneous Fat. Meat Sci. 2020, 160, 107945.
- Jerónimo, E.; Soldado, D.; Sengo, S.; Francisco, A.; Fernandes, F.; Portugal, A.P.V.; Alves, S.P.; Santos-Silva, J.; Bessa, R.J.B. Increasing the α-Tocopherol Content and Lipid Oxidative Stability of Meat through Dietary Cistus ladanifer L. in Lamb Fed Increasing Levels of Polyunsaturated Fatty Acid Rich Vegetable Oils. Meat Sci. 2020, 164, 108092.
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose Biorefineries: A Review on Biomass Pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864.
- Patel, S.; Goyal, A. Functional Oligosaccharides: Production, Properties and Applications. World J. Microbiol. Biotechnol. 2011, 27, 1119–1128.
- Lopes, T.F.; Carvalheiro, F.; Duarte, L.C.; Gírio, F.; Quintero, J.A.; Aroca, G. Techno-Economic and Life-Cycle Assessments of Small-Scale Biorefineries for Isobutene and Xylo-Oligosaccharides Production: A Comparative Study in Portugal and Chile. Biofuels Bioprod. Biorefining 2019, 13, 1321–1332.
- Alves-Ferreira, J.; Duarte, L.C.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Hydrothermal Treatments of Cistus ladanifer Industrial Residues Obtained from Essential Oil Distilleries. Waste Biomass Valorization 2019, 10, 1303–1310.
- Alves-Ferreira, J.; Duarte, L.C.; Lourenço, A.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Distillery Residues from Cistus ladanifer (Rockrose) as Feedstock for the Production of Added-Value Phenolic Compounds and Hemicellulosic Oligosaccharides. Bioenergy Res. 2019, 12, 347–358.
- Alves-Ferreira, J.; Miranda, I.; Duarte, L.C.; Roseiro, L.B.; Lourenço, A.; Quilhó, T.; Cardoso, S.; Fernandes, M.C.; Carvalheiro, F.; Pereira, H. Cistus ladanifer as a Source of Chemicals: Structural and Chemical Characterization. Biomass Convers. Biorefinery 2020, 10, 325–337.
- Ferro, M.D.; Fernandes, M.C.; Paulino, A.F.C.; Prozil, S.O.; Gravitis, J.; Evtuguin, D.V.; Xavier, A.M. Bioethanol Production from Steam Explosion Pretreated and Alkali Extracted Cistus ladanifer (Rockrose). Biochem. Eng. J. 2015, 104, 98–105.
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 7.
- Alves-Ferreira, J.; Lourenço, A.; Morgado, F.; Duarte, L.C.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Delignification of Cistus ladanifer Biomass by Organosolv and Alkali Processes. Energies 2021, 14, 1127.
- Fernandes, M.C.; Alves-Ferreira, J.; Duarte, L.C.; Pereira, H.; Carvalheiro, F.; Martínez, A. D-Lactic Acid Production from Hydrothermally Pretreated, Alkali Delignified and Enzymatically Saccharified Rockrose with the Metabolic Engineered Escherichia coli Strain JU15. Biomass Convers. Biorefin. 2022, 1–10.
- Alves-Ferreira, J.; Carvalheiro, F.; Duarte, L.C.; Ferreira, A.R.P.; Martinez, A.; Pereira, H.; Fernandes, M.C. D-Lactic Acid Production from Cistus Ladanifer Residues: Co-Fermentation of Pentoses and Hexoses by Escherichia coli JU15. Ind. Crops Prod. 2022, 177, 114519.
