Cerebrospinal fluid (CSF) is the liquid that fills the brain ventricles. CSF represents not only a mechanical brain protection but also a rich source of signalling factors modulating diverse processes during brain development and adulthood. The choroid plexus (CP) is a major source of CSF and as such it has recently emerged as an important mediator of extracellular signalling within the brain. Growing interest in the CP revealed its capacity to release a broad variety of bioactive molecules that, via CSF, regulate processes across the whole central nervous system (CNS). Moreover, CP has been also recognized as a sensor, responding to altered composition of CSF associated with changes in the patterns of CNS activity. In this review, we summarize the recent advances in our understanding of the CP as a signalling centre that mediates long-range communication in the CNS. By providing a detailed account of the CP secretory repertoire, we describe how the CP contributes to the regulation of the extracellular environment—in the context of both the embryonal as well as the adult CNS. We highlight the role of the CP as an important regulator of CNS function that acts via CSF-mediated signalling. Further studies of CP–CSF signalling hold the potential to provide key insights into the biology of the CNS, with implications for better understanding and treatment of neuropathological conditions.
- cerebrospinal fluid
- choroid plexus
- secretion
1. Introduction
The choroid plexus (CP) is a secretory tissue protruding into the lumen of all brain ventricles, namely the lateral ventricle CP (LV CP), the 3rd ventricle CP, and 4th ventricle CP (4V CP), in the form of a sheet of epithelial cells that are in direct contact with the CSF and encapsulate richly-vascularized stroma [1]. Unlike other developing processes, CP development progresses in a posterior to anterior manner with 4V CP being first to develop, followed by LV CP with 3V CP being last to emerge [2]. The CP arises from progenitor cells, specified early in the development [3], that are distributed along the dorsal midline and rhombic lip in the case of 4V CP [4][5]. CP epithelium (CPe), which originates in the neuroectoderm [6], forms a monolayer of polarized cuboidal cells with high expression of various transport proteins indicating robust secretory capacity [7][8]. Signalling from the CPe is instrumental for the induction of differentiation of the underlying CP mesenchyme and their mutual interaction is further required for proper choroid plexus morphogenesis [9][10]. Moreover, the CP is populated by additional cell types including immune cells and neurons, indicative of CP functional versatility [11][12].
As such, the CP represents a complex tissue that fulfils distinct roles essential to the CNS function. Several lines of evidence clearly established the CP as the major site for CSF production [13], despite some controversy still remaining regarding the extent of its contribution [14]. Importantly, ablation of various channel and transporter proteins located at the apical side of the CPe resulted in severe decrease in the CSF production providing compelling evidence for role of the CP in this process. Furthermore, the CP has been implicated in the CNS homeostasis via maintenance of CSF pH balance and ion osmoregulation [8]. Along the same lines, the CP actively contributes to the removal of harmful compounds originating from the blood stream or generated by brain metabolism [15][16].
2. Features and Influences
The key functional feature of the CP, conferred by the presence of junction proteins in the epithelium [17][7], is the ability of the CPe to act as a selectively-permeable interface, preventing free passage of compounds between CSF and the blood, thus establishing the blood–CSF barrier (BCSFB) [18]. This functional aspect of CP biology is essential. Fenestrated capillaries in the CP stroma and substantial local blood flow rate collectively create a highly-permeable environment enabling fast and unhindered spread of substances from blood to the CP stroma [19]. Significant protein secretion capacity displayed by CPe in tandem with selective transport of compounds from the blood stream might explain the differences of proteomic profiles between CSF and blood [20][21]. Due to its convoluted morphology and presence of microvilli on the apical surface, CPe surface area corresponds up to 50% of the overall luminal area of brain capillaries establishing the blood–brain barrier (BBB) [22][23]. Upon their maturation, CP epithelial cells manifest increased mitochondrial density, thought to provide energy supply for the considerable metabolic demands linked to the secretory activity of the CPe [23][24].
Despite shared morphology and function, embryonic CPs preserve their specific domain identities. They reflect position of the CP along the midline axis and underlie distinct transcription signatures and heterogeneous proteomic profiles observed between different embryonic CPs [11][25]. Intriguingly, secretome differences revealed across embryonic CPs are suggestive of spatially specific gradients of signalling molecules that lead to the localized activation of downstream signalling pathways within the brain. This site-specific effect of various CP-derived regulators further combines with the compartmentalization of CSF flow within the ventricular system caused by ciliary beating or bodily movements [26]. Indeed, SHH and Wnt-5a ligands, both selectively enriched in the embryonic 4V CP, have been recently linked to the modulation of proliferation and tissue patterning in the adjacent cerebellum [27][28]. Importantly, regionalized proteomic profiles may also underlie morphological differences between individual CPs as they have been implicated in different aspects of tissue morphogenesis such as the maintenance of specific progenitor domain associated with the embryonic 4V CP-derived SHH or control of epithelial branching via action of Wnt-5a [29][30]. In addition, observed molecular heterogeneity is associated not only with embryonic epithelial cells but was identified also in other cell populations of developing CPs including fibroblasts, possibly adding another layer to the complexity and specificity of the CP secretory repertoire [11]. Of interest, domain-specific differences in molecular make-up of CPs are also preserved in adulthood. For example, Sod3 gene expression, encoding a metabolic enzyme, is limited to the adult 4V CP, whereas the expression pattern for protein kinase encoded by the Penk gene, is completely reversed [25]. The age-dependent shift in the expression of various genes underlying CP detoxification or CSF production capacity has also been observed [7][15], revealing the dynamic nature of the CP secretory profile over time. It, however, seems that the importance of CSF-borne bioactive molecules released by CSF gradually decreases with age. This view is supported by the general decline in the CPe gene expression in adulthood and the gradual reduction in the CSF vs. brain tissue ratio [17][25]. There have also been recent findings showing suppressed ability of CSF to promote neurogenesis correlated with age-dependent changes in the CP secretome [31][32]. Interestingly, secreted protein Klotho associated with significant anti-aging properties is highly expressed by the CP during early development and adulthood and its CSF levels exhibit gradual decrease during aging [33][34]. Overall, it is possible that this altered pattern of CP secretory activity may reflect more general changes in CNS biology at different stages of life.
Another emerging aspect of CP function is the intrinsic ability to sense and respond to changes in the CSF as well as broader physiological changes. It has been recently shown that the CP expresses genes encoding components of circadian clock machinery, such as Bma1, Per1, and Per2, which allow the CP to influence activity of the key hypothalamic centre involved in the sleep/wake rhythmicity via secreted signals carried by CSF [35][36]. Remarkably, this mode of circadian clock regulation displays sex differences mediated by estrogen signalling [37], which is in line with the previous findings showing sex-based variability in the CP gene expression profile and proteomic signature [38]. It is noteworthy that it has been recently reported that the CP might be, at least partially, involved in the contextual fear-learning as it exhibits, in some instances, stronger response to stressful stimuli at the levels of gene transcription as compared to the hippocampus. Altered expression of multiple genes encoding secreted molecules such as the putative hormone augurin represent an example [39][40]. Recently, the CP has been also implicated as an entry site for various hormones produced in response to changed physiological state that are present in the blood, thus directly affecting their availability in the brain. For example, expression of the receptor for the peptide hormone leptin in the CP, which is involved in the regulation of the fat balance in the body, has been shown to be the limiting step, determining the transport rate of leptin from the blood stream into the CSF [41].
Due to the expression of specific receptors, the CP has been also shown to respond to the presence of neurotransmitters present in CSF such as serotonin or nicotine, which are able to elicit robust changes in the CP metabolism and transcriptome [42][43]. In addition, a recent pioneering study, leveraging a new technique for real-time monitoring of CP activity allowed characterization of a novel mode of apocrine secretion from the CPe in response to stimulation via a serotonin receptor agonist [44].
CSF plays an important role as the key modulator of neuroinflammation. CSF contains distinct pools of activated immune cells, which can be enriched in various neurodegenerative diseases such as Alzheimer’s disease (AD) [45][46]. Moreover, CSF displays a complex profile of cytokines and chemokines, which changes dynamically in different neuropathological conditions [47][48]. Interestingly, CSF-mediated regulation of neuroinflammatory response is shaped by the glymphatic system that serves as an important route for drainage and active clearance of immunomodulators and immune cells present in the CSF [49]. Given the profound changes of the adult CP transcriptome in response to inflammatory stimuli, the CP has recently emerged as an active sensor participating in immunosurveillance within the brain that is capable of dramatically altering CSF proteome via active secretion of cytokines or metallopeptidases [20][50][51]. The CP has been also suggested as the primary site for the initiation of CNS inflammation, allowing free passage of immunocompetent cells from the blood into the CP stroma and their ensuing infiltration of the CSF [44][52][53]. This process is mediated by the upregulation of locally-secreted factors forming gradients, homing immune cells towards the CP epithelium, which exhibits disrupted organization allowing their paracellular passage into the CSF [54][55][56]. Interestingly, upon inflammation, leukocytes present in the CSF can invade the CP, suggesting the possibility of two-way trafficking of immunocompetent cells across the CP epithelium [57]. Considering the scope of effects associated with the CP-mediated secretion of immunomodulators and its role in the facilitation of leukocyte entry into the brain [58], the CP has been established as a central regulator of neuroinflammatory processes within the brain, raising important questions regarding the immune privilege of the CNS.
By virtue of its strategic location at the centre of the brain ventricular system, possession of barrier-like properties enabling tight control over CSF content, close contact with blood-borne signals, robust secretory capacity, and ability to sense changes in the local environment, the CP is uniquely poised to act as a master regulator of long-range signalling in the CNS. The CP thus acts as a principal nexus for integration and transmission of signals along the brain–body axis.
3. Concluding Remarks
Direct contact between neurogenic brain regions and CSF is the hallmark of CNS biology throughout life. CSF is the chief source of trophic factors and instructive cues underlying the key aspects of embryonic development and CNS patterning. The importance of CSF is also conserved in adulthood, when it plays a key role in the regulation of adult neurogenesis and the perturbation of its content is involved in numerous pathological conditions. Considering major breakthroughs in our understanding of CP function, it is becoming increasingly evident that the CP is the major player regulating signalling properties of CSF. This view is further emphasized by a growing list of signalling factors and transporting vesicles either directly produced in the CP or actively transferred from blood across the CP, which acts as a selective barrier between blood circulation and CSF (summarized in Figure 1). Importantly, these factors and vesicles have been linked to a myriad of aspects of brain biology during development and in adulthood. As a result of age progression, active infections or changes of physiological states, CP transcriptome and secretome can undergo dramatic changes, thus highlighting the CP as a vital component involved in the modulation of crucial biological processes. Considering the overarching influence of the CP as the signalling hub of the brain, the recent emergence of experimental approaches for closer examination and manipulation of various facets of CP secretory activity promises to shed light on various outstanding challenges facing the field. Virus-based vectors, have been described as an exciting new tool for targeted and highly efficient gene delivery, enabling gene manipulation in the CP [59] and providing a powerful technique for modulation of CNS biological functions via specific alterations of CP proteome [60]. In addition, there is growing scientific interest in leveraging the potential of exosomes and lipoproteins for brain-targeted drug delivery [61][62]. At the same time, organoids have been recently recognized as an interesting model to study development of the CP and CSF production [2][63], as evidenced by presence of functional CP-like structures connected to fluid-filled cavities mimicking the functional CP–CSF interface [64][65]. Given the possibility of genetic manipulation, organoids represent a tractable model for the investigation of the molecular mechanism underlying various pathologies associated with impaired CP secretion and CSF production [66][67][68]. Thus, examination of CP secretory properties using a wide array of newly-developed molecular techniques represents an alluring avenue for future research with important implications for our understanding of brain biology across life and improvement of medical interventions aimed at the underlying causes of various developmental or neurodegenerative conditions.
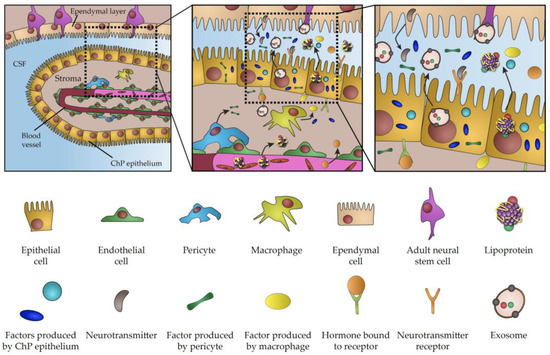
Figure 1. Schematic representation of choroid plexus secretome.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21134760
References
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457.
- Fame, R.M.; Lehtinen, M.K. Emergence and Developmental Roles of the Cerebrospinal Fluid System. Dev. Cell 2020, 52, 261–275.
- Thomas, T.; Dziadek, M. Capacity to form choroid plexus-like cells in vitro is restricted to specific regions of the mouse neural ectoderm. Development 1993, 117, 253–262.
- Currle, D.S.; Cheng, X.; Hsu, C.; Monuki, E.S. Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development 2005, 132, 3549–3559.
- Hunter, N.L.; Dymecki, S.M. Molecularly and temporally separable lineages form the hindbrain roof plate and contribute differentially to the choroid plexus. Development 2007, 134, 3449–3460.
- Awatramani, R.; Soriano, P.; Rodriguez, C.; Mai, J.J.; Dymecki, S.M. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat. Genet. 2003, 35, 70–75.
- Liddelow, S.A.; Temple, S.; Møllgård, K.; Gehwolf, R.; Wagner, A.; Bauer, H.; Bauer, H.-C.; Phoenix, T.N.; Dziegielewska, K.M.; Saunders, N.R. Molecular Characterisation of Transport Mechanisms at the Developing Mouse Blood–CSF Interface: A Transcriptome Approach. PLoS ONE 2012, 7, e33554.
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal Fluid Secretion by the Choroid Plexus. Physiol. Rev. 2013, 93, 1847–1892.
- Nielsen, C.M.; Dymecki, S.M. Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev. Biol. 2010, 340, 430–437.
- Wilting, J.; Christ, B. An experimental and ultrastructural study on the development of the avian choroid plexus. Cell Tissue Res. 1989, 255, 487–494.
- Dani, N.; Herbst, R.H.; Habib, N.; Head, J.; Dionne, D.; Nguyen, L.; McCabe, C.; Cui, J.; Shipley, F.B.; Jang, A.; et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. bioRxiv 2019, 627539.
- Prasongchean, W.; Vernay, B.; Asgarian, Z.; Jannatul, N.; Ferretti, P. The neural milieu of the developing choroid plexus: Neural stem cells, neurons and innervation. Front. Neurosci. 2015, 9, 103.
- Spector, R.; Keep, R.F.; Robert Snodgrass, S.; Smith, Q.R.; Johanson, C.E. A balanced view of choroid plexus structure and function: Focus on adult humans. Exp. Neurol. 2015, 267, 78–86.
- Orešković, D. The controversy on choroid plexus function in cerebrospinal fluid production in humans: How long different views could be neglected? Croat. Med. J. 2015, 56, 306–310.
- Kratzer, I.; Strazielle, N.; Saudrais, E.; Mönkkönen, K.; Malleval, C.; Blondel, S.; Ghersi-Egea, J.-F. Glutathione Conjugation at the Blood-CSF Barrier Efficiently Prevents Exposure of the Developing Brain Fluid Environment to Blood-Borne Reactive Electrophilic Substances. J. Neurosci. 2018, 38, 3466–3479.
- Saudrais, E.; Strazielle, N.; Ghersi-Egea, J.-F. Choroid plexus glutathione peroxidases are instrumental in protecting the brain fluid environment from hydroperoxides during postnatal development. Am. J. Physiol. Physiol. 2018, 315, C445–C456.
- Ghersi-Egea, J.-F.; Babikian, A.; Blondel, S.; Strazielle, N. Changes in the cerebrospinal fluid circulatory system of the developing rat: Quantitative volumetric analysis and effect on blood-CSF permeability interpretation. Fluids Barriers CNS 2015, 12, 8.
- Ghersi-Egea, J.F.; Strazielle, N.; Catala, M.; Silva-Vargas, V.; Doetsch, F.; Engelhardt, B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018, 135, 337–361.
- Strazielle, N.; Ghersi-Egea, J.F. Physiology of Blood–Brain Interfaces in Relation to Brain Disposition of Small Compounds and Macromolecules. Mol. Pharm. 2013, 10, 1473–1491.
- Thouvenot, E.; Lafon-Cazal, M.; Demettre, E.; Jouin, P.; Bockaert, J.; Marin, P. The proteomic analysis of mouse choroid plexus secretome reveals a high protein secretion capacity of choroidal epithelial cells. Proteomics 2006, 6, 5941–5952.
- Guldbrandsen, A.; Vethe, H.; Farag, Y.; Oveland, E.; Garberg, H.; Berle, M.; Myhr, K.-M.; Opsahl, J.A.; Barsnes, H.; Berven, F.S. In-depth Characterization of the Cerebrospinal Fluid (CSF) Proteome Displayed Through the CSF Proteome Resource (CSF-PR). Mol. Cell. Proteomics 2014, 13, 3152–3163.
- Keep, R.F.; Jones, H.C. A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Dev. Brain Res. 1990, 56, 47–53.
- Cornford, E.M.; Varesi, J.B.; Hyman, S.; Damian, R.T.; Raleigh, M.J. Mitochondrial content of choroid plexus epithelium. Exp. Brain Res. 1997, 116, 399–405.
- Sturrock, R.R. A morphological study of the development of the mouse choroid plexus. J. Anat. 1979, 129, 777–793.
- Lun, M.P.; Johnson, M.B.; Broadbelt, K.G.; Watanabe, M.; Kang, Y.-j.; Chau, K.F.; Springel, M.W.; Malesz, A.; Sousa, A.M.M.; Pletikos, M.; et al. Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production. J. Neurosci. 2015, 35, 4903–4916.
- Olstad, E.W.; Ringers, C.; Hansen, J.N.; Wens, A.; Brandt, C.; Wachten, D.; Yaksi, E.; Jurisch-Yaksi, N. Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development. Curr. Biol. 2019, 29, 229–241.e6.
- Kaiser, K.; Gyllborg, D.; Procházka, J.; Salašová, A.; Kompaníková, P.; Molina, F.L.; Laguna-Goya, R.; Radaszkiewicz, T.; Harnoš, J.; Procházková, M.; et al. WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis. Nat. Commun. 2019, 10, 1–15.
- Huang, X.; Liu, J.; Ketova, T.; Fleming, J.T.; Grover, V.K.; Cooper, M.K.; Litingtung, Y.; Chiang, C. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc. Natl. Acad. Sci. USA 2010, 107, 8422–8427.
- Huang, X.; Ketova, T.; Fleming, J.T.; Wang, H.; Dey, S.K.; Litingtung, Y.; Chiang, C. Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus. Development 2009, 136, 2535–2543.
- Kaiser, K.; Jang, A.; Lun, M.; Prochazka, J.; Machon, O.; Prochazkova, M.; Laurent, B.; Gyllborg, D.; van Amerongen, R.; Kompanikova, P.; et al. MEIS-WNT5A axis regulates development of 4th ventricle choroid plexus. bioRxiv 2020.
- Lehtinen, M.K.; Zappaterra, M.W.; Chen, X.; Yang, Y.J.; Hill, A.D.; Lun, M.; Maynard, T.; Gonzalez, D.; Kim, S.; Ye, P.; et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 2011, 69, 893–905.
- Silva-Vargas, V.; Maldonado-Soto, A.R.; Mizrak, D.; Codega, P.; Doetsch, F. Age-Dependent Niche Signals from the Choroid Plexus Regulate Adult Neural Stem Cells. Cell Stem Cell 2016, 19, 643–652.
- Vo, H.T.; Laszczyk, A.M.; King, G.D. Klotho, the Key to Healthy Brain Aging? Brain Plast. 2018, 3, 183–194.
- Semba, R.D.; Moghekar, A.R.; Hu, J.; Sun, K.; Turner, R.; Ferrucci, L.; O’Brien, R. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci. Lett. 2014, 558, 37–40.
- Myung, J.; Schmal, C.; Hong, S.; Tsukizawa, Y.; Rose, P.; Zhang, Y.; Holtzman, M.J.; De Schutter, E.; Herzel, H.; Bordyugov, G.; et al. The choroid plexus is an important circadian clock component. Nat. Commun. 2018, 9, 1062.
- Quintela, T.; Sousa, C.; Patriarca, F.M.; Gonçalves, I.; Santos, C.R.A. Gender associated circadian oscillations of the clock genes in rat choroid plexus. Brain Struct. Funct. 2015, 220, 1251–1262.
- Quintela, T.; Albuquerque, T.; Lundkvist, G.; Carmine Belin, A.; Talhada, D.; Gonçalves, I.; Carro, E.; Santos, C.R.A. The choroid plexus harbors a circadian oscillator modulated by estrogens. Chronobiol. Int. 2018, 35, 270–279.
- Quintela, T.; Marcelino, H.; Deery, M.J.; Feret, R.; Howard, J.; Lilley, K.S.; Albuquerque, T.; Gonçalves, I.; Duarte, A.C.; Santos, C.R.A. Sex-Related Differences in Rat Choroid Plexus and Cerebrospinal Fluid: A cDNA Microarray and Proteomic Analysis. J. Neuroendocrinol. 2016, 28.
- Cho, J.; Yu, N.-K.; Choi, J.-H.; Sim, S.-E.; Kang, S.J.; Kwak, C.; Lee, S.-W.; Kim, J.; Choi, D.I.; Kim, V.N.; et al. Multiple repressive mechanisms in the hippocampus during memory formation. Science 2015, 350, 82–87.
- Mathew, R.S.; Mullan, H.; Blusztajn, J.K.; Lehtinen, M.K. Comment on “Multiple repressive mechanisms in the hippocampus during memory formation”. Science 2016, 353, 453.
- Harrison, L.; Schriever, S.C.; Feuchtinger, A.; Kyriakou, E.; Baumann, P.; Pfuhlmann, K.; Messias, A.C.; Walch, A.; Tschöp, M.H.; Pfluger, P.T. Fluorescent blood–brain barrier tracing shows intact leptin transport in obese mice. Int. J. Obes. 2019, 43, 1305–1318.
- Lallai, V.; Grimes, N.; Fowler, J.P.; Sequeira, P.A.; Cartagena, P.; Limon, A.; Coutts, M.; Monuki, E.S.; Bunney, W.; Demuro, A.; et al. Nicotine Acts on Cholinergic Signaling Mechanisms to Directly Modulate Choroid Plexus Function. eNeuro 2019, 6.
- Conn, P.J.; Sanders-Bush, E.; Hoffman, B.J.; Hartig, P.R. A unique serotonin receptor in choroid plexus is linked to phosphatidylinositol turnover. Proc. Natl. Acad. Sci. USA 1986, 83, 4086–4088.
- Shipley, F.B.; Dani, N.; Xu, H.; Deister, C.; Cui, J.; Head, J.P.; Sadegh, C.; Fame, R.M.; Shannon, M.L.; Flores, V.I.; et al. Tracking Calcium Dynamics and Immune Surveillance at the Choroid Plexus Blood-Cerebrospinal Fluid Interface. Neuron 2020.
- Liu, Y.; He, X.; Li, Y.; Wang, T. Cerebrospinal fluid CD4+ T lymphocyte-derived miRNA-let-7b can enhances the diagnostic performance of Alzheimer’s disease biomarkers. Biochem. Biophys. Res. Commun. 2018, 495, 1144–1150.
- Kivisäkk, P.; Mahad, D.J.; Callahan, M.K.; Trebst, C.; Tucky, B.; Wei, T.; Wu, L.; Baekkevold, E.S.; Lassmann, H.; Staugaitis, S.M.; et al. Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc. Natl. Acad. Sci. USA 2003, 100, 8389–8394.
- Meeker, R.B.; Poulton, W.; Markovic-Plese, S.; Hall, C.; Robertson, K. Protein changes in CSF of HIV-infected patients: Evidence for loss of neuroprotection. J. Neurovirol. 2011, 17, 258–273.
- Kothur, K.; Wienholt, L.; Brilot, F.; Dale, R.C. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine 2016, 77, 227–237.
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391.
- Dahm, T.; Frank, F.; Adams, O.; Lindner, H.A.; Ishikawa, H.; Weiss, C.; Schwerk, C.; Schroten, H.; Tenenbaum, T.; Rudolph, H. Sequential transmigration of polymorphonuclear cells and naive CD3+ T lymphocytes across the blood-cerebrospinal-fluid barrier in vitro following infection with Echovirus 30. Virus Res. 2017, 232, 54–62.
- Marques, F.; Sousa, J.C.; Coppola, G.; Falcao, A.M.; Rodrigues, A.J.; Geschwind, D.H.; Sousa, N.; Correia-Neves, M.; Palha, J.A. Kinetic Profile of the Transcriptome Changes Induced in the Choroid Plexus by Peripheral Inflammation. J. Cereb. Blood Flow Metab. 2009, 29, 921–932.
- Endo, H.; Sasaki, K.; Tonosaki, A.; Kayama, T. Three-dimensional and ultrastructural ICAM-1 distribution in the choroid plexus, arachnoid membrane and dural sinus of inflammatory rats induced by LPS injection in the lateral ventricles. Brain Res. 1998, 793, 297–301.
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009, 10, 514–523.
- Mottahedin, A.; Joakim Ek, C.; Truvé, K.; Hagberg, H.; Mallard, C. Choroid plexus transcriptome and ultrastructure analysis reveals a TLR2-specific chemotaxis signature and cytoskeleton remodeling in leukocyte trafficking. Brain. Behav. Immun. 2019, 79, 216–227.
- Mottahedin, A.; Blondel, S.; Ek, J.; Leverin, A.-L.; Svedin, P.; Hagberg, H.; Mallard, C.; Ghersi-Egea, J.-F.; Strazielle, N. N-acetylcysteine inhibits bacterial lipopeptide-mediated neutrophil transmigration through the choroid plexus in the developing brain. Acta Neuropathol. Commun. 2020, 8, 4.
- Strazielle, N.; Creidy, R.; Malcus, C.; Boucraut, J.; Ghersi-Egea, J.-F. T-Lymphocytes Traffic into the Brain across the Blood-CSF Barrier: Evidence Using a Reconstituted Choroid Plexus Epithelium. PLoS ONE 2016, 11, e0150945.
- Strominger, I.; Elyahu, Y.; Berner, O.; Reckhow, J.; Mittal, K.; Nemirovsky, A.; Monsonego, A. The Choroid Plexus Functions as a Niche for T-Cell Stimulation Within the Central Nervous System. Front. Immunol. 2018, 9, 1066.
- Demeestere, D.; Libert, C.; Vandenbroucke, R.E. Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders. Drug Discov. Today 2015, 20, 928–941.
- Chen, X.; He, Y.; Tian, Y.; Wang, Y.; Wu, Z.; Lan, T.; Wang, H.; Cheng, K.; Xie, P. Different Serotypes of Adeno-Associated Virus Vector- and Lentivirus-Mediated Tropism in Choroid Plexus by Intracerebroventricular Delivery. Hum. Gene Ther. 2020, 31, 440–447.
- Regev, L.; Ezrielev, E.; Gershon, E.; Gil, S.; Chen, A. Genetic approach for intracerebroventricular delivery. Proc. Natl. Acad. Sci. USA 2010, 107, 4424–4429.
- Zheng, M.; Huang, M.; Ma, X.; Chen, H.; Gao, X. Harnessing Exosomes for the Development of Brain Drug Delivery Systems. Bioconjug. Chem. 2019, 30, 994–1005.
- Zhu, C.; Xia, Y. Biomimetics: Reconstitution of low-density lipoprotein for targeted drug delivery and related theranostic applications. Chem. Soc. Rev. 2017, 46, 7668–7682.
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597.
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 2020, eaaz5626.
- Renner, M.; Lancaster, M.A.; Bian, S.; Choi, H.; Ku, T.; Peer, A.; Chung, K.; Knoblich, J.A. Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 2017, 36, 1316–1329.
- Coulter, M.E.; Dorobantu, C.M.; Lodewijk, G.A.; Delalande, F.; Cianferani, S.; Ganesh, V.S.; Smith, R.S.; Lim, E.T.; Xu, C.S.; Pang, S.; et al. The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles. Cell Rep. 2018, 24, 973–986.e8.
- Hochstetler, A.E.; Whitehouse, L.; Antonellis, P.; Berbari, N.F.; Blazer-Yost, B.L. Characterizing the Expression of TRPV4 in the Choroid Plexus Epithelia as a Prospective Component in the Development of Hydrocephalus in the Gas8GT Juvenile Mouse Model. FASEB J. 2018, 32, 750.12.
- Allocco, A.A.; Jin, S.C.; Duy, P.Q.; Furey, C.G.; Zeng, X.; Dong, W.; Nelson-Williams, C.; Karimy, J.K.; DeSpenza, T.; Hao, L.T.; et al. Recessive Inheritance of Congenital Hydrocephalus With Other Structural Brain Abnormalities Caused by Compound Heterozygous Mutations in ATP1A3. Front. Cell. Neurosci. 2019, 13, 425.
