Mature B cells notably diversify immunoglobulin (Ig) production through class switch recombination (CSR), allowing the junction of distant “switch” (S) regions. CSR is initiated by activation-induced deaminase (AID), which targets cytosines adequately exposed within single-stranded DNA of transcribed targeted S regions, with a specific affinity for WRCY motifs. In mammalian S regions, abundant G4 (G-quadruplex) DNA on the non-template strand also contributes to the formation of R-loops while the presence of G4 structures within the primary transcripts from S regions participates into recruiting AID. The ability of G4 ligands to modulate the CSR process also underlines the key role of G4 structures in the regulation of CSR.
- G-quadruplex DNA
- G4-ligand
- class switch recombination
1. Role of G4-DNA at Transcribed S Regions and in the Resolution of G4s/R-Loops Conflicts
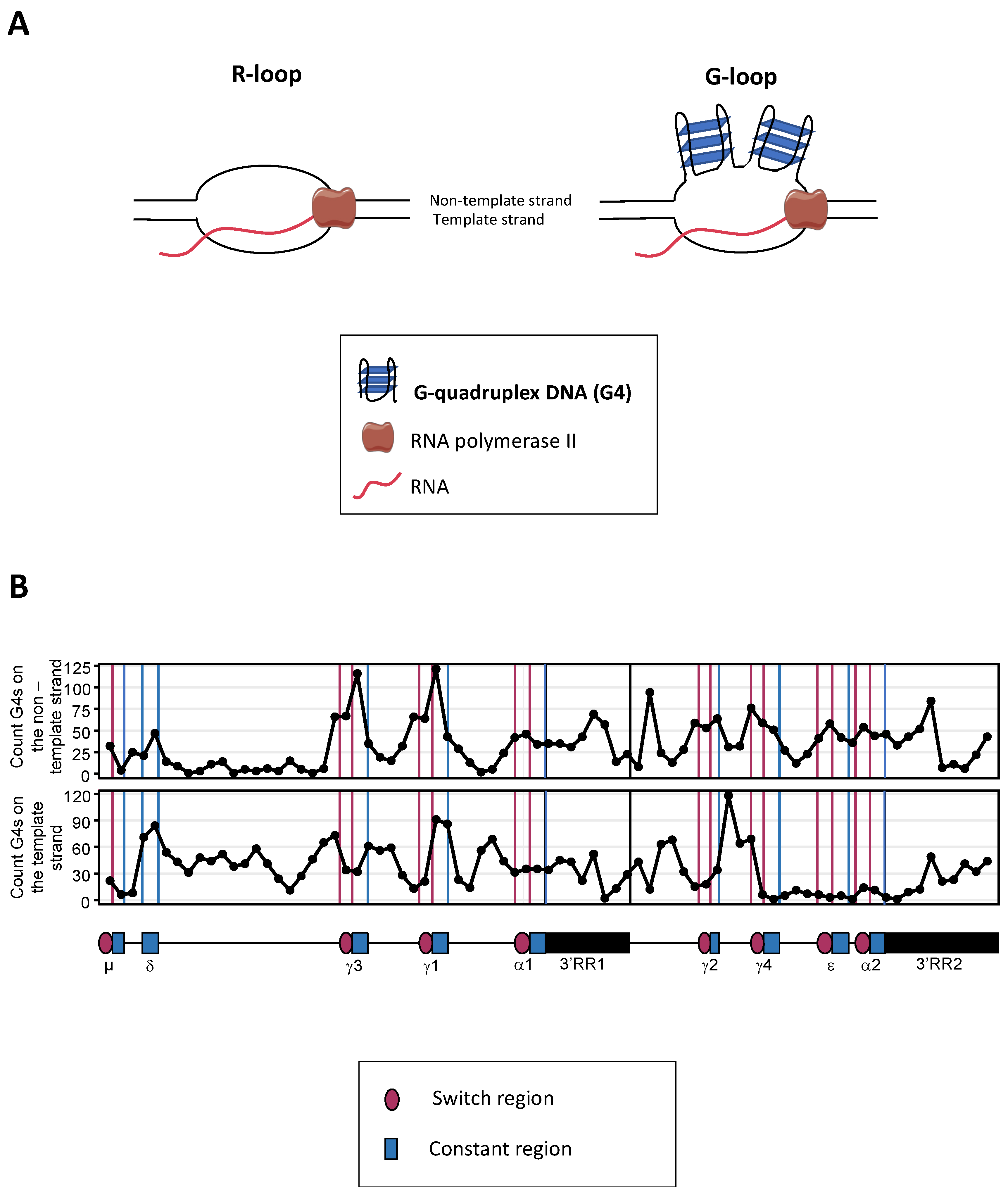
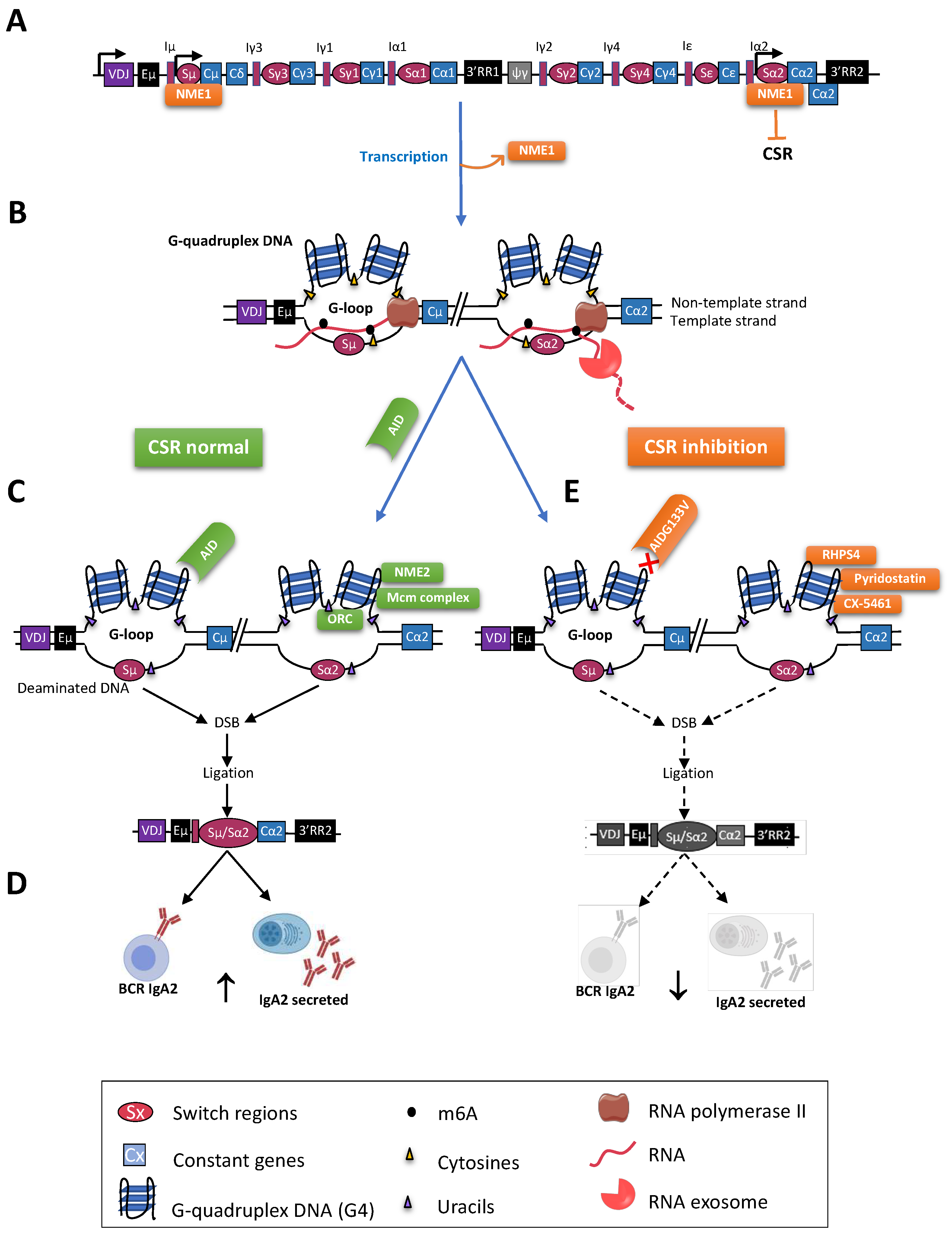
2. Role of G4-RNA Structures within S Region Transcripts
3. Connections between CSR and DNA Replication
4. A Role of G4-DNA in IgH Locus High-Dimensional Organization
This entry is adapted from the peer-reviewed paper 10.3390/molecules28031159
References
- Williamson, J.R.; Raghuraman, M.K.; Cech, T.R. Monovalent cation-induced structure of telomeric DNA: The G-quartet model. Cell 1989, 59, 871–880.
- Dalloul, Z.; Chenuet, P.; Dalloul, I.; Boyer, F.; Aldigier, J.-C.; Laffleur, B.; El Makhour, Y.; Ryffel, B.; Quesniaux, V.F.; Togbé, D.; et al. G-quadruplex DNA targeting alters class-switch recombination in B cells and attenuates allergic inflammation. J. Allergy Clin. Immunol. 2018, 142, 1352–1355.
- Qiao, Q.; Wang, L.; Meng, F.-L.; Hwang, J.K.; Alt, F.W.; Wu, H. AID Recognizes Structured DNA for Class Switch Recombination. Mol. Cell 2017, 67, 361–373.
- Vallur, A.C.; Maizels, N. Activities of human exonuclease 1 that promote cleavage of transcribed immunoglobulin switch regions. Proc. Natl. Acad. Sci. USA 2008, 105, 16508–16512.
- Collier, D.A.; Griffin, J.A.; Wells, R.D. Non-B right-handed DNA conformations of homopurine.homopyrimidine sequences in the murine immunoglobulin C alpha switch region. J. Biol. Chem. 1988, 263, 7397–7405.
- Sen, D.; Gilbert, W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature 1988, 334, 364–366.
- Chi, X.; Li, Y.; Qiu, X. V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: Mechanism and regulation. Immunology 2020, 160, 233–247.
- Abbott, R.K.; Thayer, M.; Labuda, J.; Silva, M.; Philbrook, P.; Cain, D.W.; Kojima, H.; Hatfield, S.; Sethumadhavan, S.; Ohta, A.; et al. Germinal Center Hypoxia Potentiates Immunoglobulin Class Switch Recombination. J. Immunol. 2016, 197, 4014–4020.
- Cho, S.H.; Raybuck, A.L.; Stengel, K.; Wei, M.; Beck, T.C.; Volanakis, E.; Thomas, J.W.; Hiebert, S.; Haase, V.H.; Boothby, M.R. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature 2016, 537, 234–238.
- Matthews, A.J.; Zheng, S.; DiMenna, L.J.; Chaudhuri, J. Chapter One-Regulation of Immunoglobulin Class-Switch Recombination: Choreography of Noncoding Transcription, Targeted DNA Deamination, and Long-Range DNA Repair. Adv. Immunol. 2014, 122, 1–57.
- Nair, L.; Zhang, W.; Laffleur, B.; Jha, M.K.; Lim, J.; Lee, H.; Wu, L.; Alvarez, N.S.; Liu, Z.-P.; Munteanu, E.L.; et al. Mechanism of noncoding RNA-associated N6-methyladenosine recognition by an RNA processing complex during IgH DNA recombination. Mol. Cell 2021, 81, 3949–3964.
- Shinkura, R.; Tian, M.; Smith, M.; Chua, K.; Fujiwara, Y.; Alt, F.W. The influence of transcriptional orientation on endogenous switch region function. Nat. Immunol. 2003, 4, 435–441.
- Tashiro, J.; Kinoshita, K.; Honjo, T. Palindromic but not G-rich sequences are targets of class switch recombination. Int. Immunol. 2001, 13, 495–505.
- Zheng, S.; Kusnadi, A.; Choi, J.E.; Vuong, B.Q.; Rhodes, D.; Chaudhuri, J. NME proteins regulate class switch recombination. FEBS Lett. 2019, 593, 80–87.
- Guo, J.U.; Bartel, D.P. RNA G-quadruplexes are globally unfolded in eukaryotic cells and depleted in bacteria. Science 2016, 353, aaf5371.
- Kwok, C.K.; Marsico, G.; Sahakyan, A.B.; Chambers, V.S.; Balasubramanian, S. rG4-seq reveals widespread formation of G-quadruplex structures in the human transcriptome. Nat. Methods 2016, 13, 841–844.
- Yang, S.Y.; Monchaud, D.; Wong, J.M.Y. Global mapping of RNA G-quadruplexes (G4-RNAs) using G4RP-seq. Nat. Protoc. 2022, 17, 870–889.
- Yewdell, W.T.; Chaudhuri, J. A transcriptional serenAID: The role of noncoding RNAs in class switch recombination. Int. Immunol. 2017, 29, 183–196.
- Duquette, M.L.; Handa, P.; Vincent, J.A.; Taylor, A.F.; Maizels, N. Intracellular transcription of G-rich DNAs induces formation of G-loops, novel structures containing G4 DNA. Genes Dev. 2004, 18, 1618–1629.
- Fleming, A.M.; Nguyen, N.L.B.; Burrows, C.J. Colocalization of m6A and G-Quadruplex-Forming Sequences in Viral RNA (HIV, Zika, Hepatitis B, and SV40) Suggests Topological Control of Adenosine N6-Methylation. ACS Central Sci. 2019, 5, 218–228.
- Iwasaki, Y.; Ookuro, Y.; Iida, K.; Nagasawa, K.; Yoshida, W. Destabilization of DNA and RNA G-quadruplex structures formed by GGA repeat due to N6-methyladenine modification. Biochem. Biophys. Res. Commun. 2022, 597, 134–139.
- Czerniak, T.; Saenz, J.P. Lipid membranes modulate the activity of RNA through sequence-dependent interactions. Proc. Natl. Acad. Sci. USA 2022, 119, e2119235119.
- Zheng, S.; Vuong, B.Q.; Vaidyanathan, B.; Lin, J.-Y.; Huang, F.-T.; Chaudhuri, J. Non-coding RNA Generated following Lariat Debranching Mediates Targeting of AID to DNA. Cell 2015, 161, 762–773.
- De Almeida, C.R.; Dhir, S.; Dhir, A.; Moghaddam, A.E.; Sattentau, Q.; Meinhart, A.; Proudfoot, N.J. RNA Helicase DDX1 Converts RNA G-Quadruplex Structures into R-Loops to Promote IgH Class Switch Recombination. Mol. Cell 2018, 70, 650–662.
- Schrader, C.E.; Guikema, J.E.J.; Linehan, E.K.; Selsing, E.; Stavnezer, J. Activation-Induced Cytidine Deaminase-Dependent DNA Breaks in Class Switch Recombination Occur during G1 Phase of the Cell Cycle and Depend upon Mismatch Repair. J. Immunol. 2007, 179, 6064–6071.
- Wiedemann, E.-M.; Peycheva, M.; Pavri, R. DNA Replication Origins in Immunoglobulin Switch Regions Regulate Class Switch Recombination in an R-Loop-Dependent Manner. Cell Rep. 2016, 17, 2927–2942.
- Bochman, M.L.; Schwacha, A. The Mcm Complex: Unwinding the Mechanism of a Replicative Helicase. Microbiol. Mol. Biol. Rev. 2009, 73, 652–683.
- Zhang, X.; Zhang, Y.; Ba, Z.; Kyritsis, N.; Casellas, R.; Alt, F.W. Fundamental roles of chromatin loop extrusion in antibody class switching. Nature 2019, 575, 385–389.
- Zhang, Y.; Zhang, X.; Ba, Z.; Liang, Z.; Dring, E.W.; Hu, H.; Lou, J.; Kyritsis, N.; Zurita, J.; Shamim, M.S.; et al. The fundamental role of chromatin loop extrusion in physiological V(D)J recombination. Nature 2019, 573, 600–604.
- Haensel-Hertsch, R.; Beraldi, D.; Lensing, S.V.; Marsico, G.; Zyner, K.; Parry, A.; Di Antonio, M.; Pike, J.; Kimura, H.; Narita, M.; et al. G-quadruplex structures mark human regulatory chromatin. Nat. Genet. 2016, 48, 1267–1272.
- Li, L.; Williams, P.; Ren, W.; Wang, M.Y.; Gao, Z.; Miao, W.; Huang, M.; Song, J.; Wang, Y. YY1 interacts with guanine quadruplexes to regulate DNA looping and gene expression. Nat. Chem. Biol. 2021, 17, 161–168.
- Roach, R.J.; Garavís, M.; González, C.; Jameson, G.B.; Filichev, V.V.; Hale, T.K. Heterochromatin protein 1α interacts with parallel RNA and DNA G-quadruplexes. Nucleic Acids Res. 2020, 48, 682–693.
- Valton, A.-L.; Venev, S.V.; Mair, B.; Khokhar, E.S.; Tong, A.H.Y.; Usaj, M.; Chan, K.; Pai, A.A.; Moffat, J.; Dekker, J. A cohesin traffic pattern genetically linked to gene regulation. Nat. Struct. Mol. Biol. 2022, 29, 1239–1251.
- Laffleur, B.; Lim, J.; Zhang, W.; Chen, Y.; Pefanis, E.; Bizarro, J.; Batista, C.R.; Wu, L.; Economides, A.N.; Wang, J.; et al. Noncoding RNA processing by DIS3 regulates chromosomal architecture and somatic hypermutation in B cells. Nat. Genet. 2021, 53, 230–242.
- Ceschi, S.; Berselli, M.; Cozzaglio, M.; Giantin, M.; Toppo, S.; Spolaore, B.; Sissi, C. Vimentin binds to G-quadruplex repeats found at telomeres and gene promoters. Nucleic Acids Res. 2022, 50, 1370–1381.
- Wuerffel, R.; Wang, L.; Grigera, F.; Manis, J.; Selsing, E.; Perlot, T.; Alt, F.W.; Cogne, M.; Pinaud, E.; Kenter, A.L. S-S Synapsis during Class Switch Recombination Is Promoted by Distantly Located Transcriptional Elements and Activation-Induced Deaminase. Immunity 2007, 27, 711–722.
