Periodontitis is a non-communicable chronic inflammatory disease characterized by the progressive and irreversible breakdown of the soft periodontal tissues and resorption of teeth-supporting alveolar bone. The etiology of periodontitis involves dysbiotic shifts in the diversity of microbial communities inhabiting the subgingival crevice, which is dominated by anaerobic Gram-negative bacteria, including Porphyromonas gingivalis. Indeed, P. gingivalis is a keystone pathogen with a repertoire of attributes that allow it to colonize periodontal tissues and influence the metabolism, growth rate, and virulence of other periodontal bacteria. The pathogenic potential of P. gingivalis has been traditionally analyzed using classical biochemical and molecular approaches.
- periodontitis
- microbial dysbiosis
- Porphyromonas gingivalis
- keystone pathogen
1. Introduction
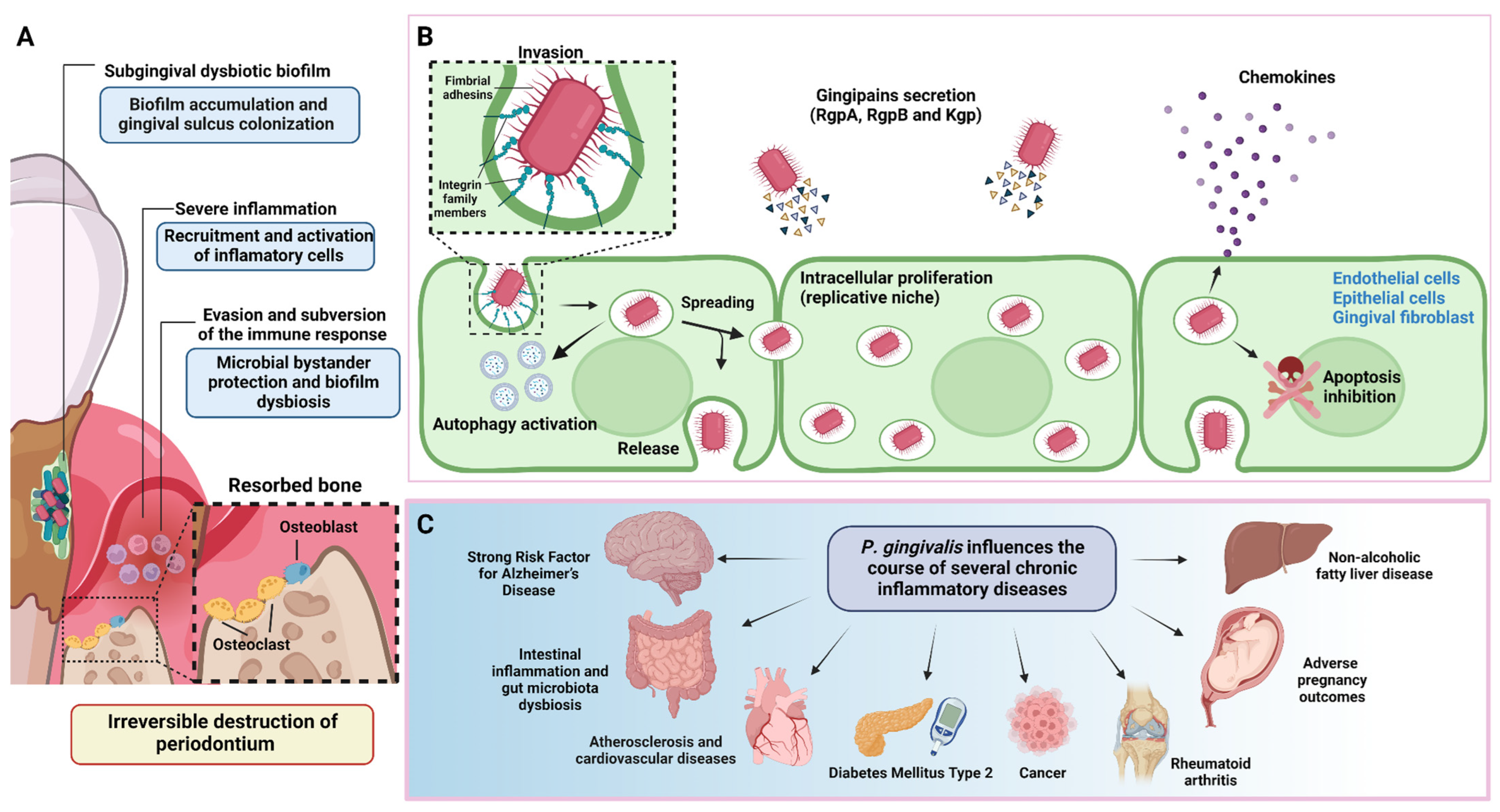
2. P. gingivalis Is a Keystone Pathogen of Periodontitis
3. Proteomics and Metabolomics in P. gingivalis Pathogenesis
| Strategy | Method and Sample | Findings/Contributions | Reference |
|---|---|---|---|
| Proteomics | Cultured P. gingivalis, F. nucleatum, and S. gordonii mixed biofilm and P. gingivalis monobiofilm as control. Bacterial cells were lysed, and proteins were digested for mass spectrometry. | The proteome of the mixed biofilm differs from the monobiofilm, it exhibits a decrease in proteins involved in cell shape and cell envelope formation, and an increase in HmuR protein (an outer membrane receptor). | [40] |
| Proteomics | Cultured P. gingivalis (ATCC®33277™) and S. oralis (ATCC®9811™) mixed biofilm. Controls are monobiofilm of P. gingivalis and S. oralis. Biofilm samples were digested and then summited to shotgun proteomic analysis with LC-MS/MS. | The P. gingivalis proteins that increased their expression induced by the interaction with S. oralis were GyrB, RpoD, FimA and a probable transcriptional regulatory protein. | [41] |
| Proteomics | Liquid cultured P. gingivalis (ATCC®33277™, W83 and two peptidylarginine deiminase (PPAD) mutant strains) were centrifugated and the supernatant was analyzed by mass spectrometry analysis. | Analysis of the P. gingivalis proteome and extracellular citrulilnome showed heterogeneity between the different isolates. Furthermore, the main virulence factors revealed different patterns in their citrullination. | [42] |
| Proteomics | Cultured P. gingivalis ATCC®33277™ and mutant strains. Cells were harvested, lysed, and the supernatant was subjected to mass spectrometry analysis. | Identification of 257 putative O-glycosylation sites within 145 glycoproteins of P. gingivalis. Demonstration for the first time the presence of the O-glycosylation system in P. gingivalis. | [43] |
| Proteomics | Cultured P. gingivalis (W50 strain), then cells were harvested and centrifuged, and the supernatant was filtered to obtain outer membrane vesicles (OMVs) for mass spectrometry analysis. | A total of 151 OMV proteins were identified and the most enriched proteins were LptO, IhtB and HmuY. | [44] |
| Proteomics | Cultured P. gingivalis (W50 strain) in three conditions: control, heme limitation, and heme excess conditions. Then cells were harvested and processed to obtain whole cell lysate and outer membrane vesicles separately and then mass spectrometry analyses. | The proteins most upregulated in response to heme limitation were those involved in binding and transporting heme. | [45] |
| Metabolomics | Tongue swabs and mouth washout samples from patients with chronic periodontal disease were analyzed with proton nuclear magnetic resonance (H-NMR) to determine their metabolic status. | The metabolic state of the mouth of chronic periodontal disease patients changes in the levels of eight metabolites in comparison to healthy individuals. These metabolic changes could be used as a periodontal disease-associated process biomarker. | [46] |
| Metabolomics | Meditation through Gas chromatography-mass spectrometer (GC-MS) metabolite profiling of cultured human periodontal ligament fibroblast infected with P. gingivalis (ATCC®33277™). | Periodontal ligament cells (PDLSCs) experienced metabolic reprogramming due to the infection of P. gingivalis. These metabolic changes could be related to pro-inflammatory responses on PDLSC, showing a shift from oxidative phosphorylation to glycolysis. | [47] |
| Metabolomics and metagenomics | Serum samples from mice submitted to an oral gavage of P. gingivalis (ATCC®33277TM) and sham control were analyzed with Untargeted metabolomics profiling chromatographic separation and mass spectrometry (MS). Additionally, RNA extraction and metagenomic analysis were done in colon samples from the same study groups. | The analysis of the metabolites in P. gingivalis-administered mice demonstrated that oral administration of this periodontal pathogen could induce dysbiosis of the gut microbiota. In addition, these derived metabolites are associated with metabolic pathways and could be related to the development of metabolic disorders and the destruction of intestinal barrier function. | [48] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms24010620
References
- Wu, Y.; Dong, G.; Xiao, W.; Xiao, E.; Miao, F.; Syverson, A.; Missaghian, N.; Vafa, R.; Ortega, A.A.C.; Rossa, J.C.; et al. Effect of aging on periodontal inflammation, microbial colonization, and disease susceptibility. J. Dent. Res. 2016, 95, 460–466.
- van Dyke, T.E.; Sima, C. Understanding resolution of inflammation in periodontal diseases: Is chronic inflammatory periodontitis a failure to resolve? Periodontology 2020, 82, 205–213.
- Graves, D.T.; Oates, T.; Garlet, G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011, 3, 5304.
- Alvarez, C.; Monasterio, G.; Cavalla, F.; Córdova, L.A.; Hernández, M.; Heymann, D.; Garlet, G.P.; Sorsa, T.; Pärnänen, P.; Lee, H.-M.; et al. Osteoimmunology of oral and maxillofacial diseases: Translational applications based on biological mechanisms. Front. Immunol. 2019, 10, 1664.
- Hajishengallis, G.; Korostoff, J.M. Revisiting the Page & Schroeder model: The good, the bad and the unknowns in the periodontal host response 40 years later. Periodontology 2017, 75, 116–151.
- Curtis, M.A.; Diaz, P.I.; van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2020, 83, 14–25.
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44.
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyronomas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53.
- Casarin, R.C.V.; Ribeiro, É.D.; Mariano, F.S.; Nociti, F.H.; Casati, M.Z.; Gonçalves, R.B. Levels of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, inflammatory cytokines and species-specific immunoglobulin G in generalized aggressive and chronic periodontitis. J. Periodontal Res. 2010, 45, 635–642.
- Griffen, A.L.; Becker, M.R.; Lyons, S.R.; Moeschberger, M.L.; Leys, E.J. Prevalence of Porphyromonas gingivalis and periodontal health status. J. Clin. Microbiol. 1998, 36, 3239–3242.
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440.
- Zenobia, C.; Hajishengallis, G. Porphyronomas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence 2015, 6, 236–243.
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.; Lambris, J.; et al. Low-Abundance Biofilm Species Orchestrates Inflammatory Periodontal Disease through the Commensal Microbiota and the Complement. Cell Host Microbe 2011, 10, 497–506.
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419.
- Tribble, G.; Kerr, J.; Wang, B. Genetic diversity in the oral pathogen Porphyronomas gingivalis: Molecular mechanisms and biological consequences. Futur. Microbiol. 2013, 8, 607–622.
- Silva, I.L.; Cascales, E. Molecular Strategies Underlying Porphyromonas gingivalis Virulence. J. Mol. Biol. 2021, 433, 166836.
- Gutleben, J.; de Mares, M.C.; van Elsas, J.D.; Smidt, H.; Overmann, J.; Sipkema, D. The multi-omics promise in context: From sequence to microbial isolate. Crit. Rev. Microbiol. 2018, 44, 212–229.
- Beale, D.J.; Kouremenos, K.A.; Palombo, E.A. Microbial Metabolomics: Applications in Clinical, Environmental, and Industrial Microbiology; Springer: Cham, Switzerland, 2016; p. 321.
- Wang, J.; Qi, J.; Zhao, H.; He, S.; Zhang, Y.; Wei, S.; Zhao, F. Metagenomic sequencing reveals microbiota and its functional potential associated with periodontal disease. Sci. Rep. 2013, 3, 1843.
- Utter, D.R.; Borisy, G.G.; Eren, A.M.; Cavanaugh, C.M.; Welch, J.L.M. Metapangenomics of the oral microbiome provides insights into habitat adaptation and cultivar diversity. Genome Biol. 2020, 21, 293.
- Williams, D.W.; Greenwell-Wild, T.; Brenchley, L.; Dutzan, N.; Overmiller, A.; Sawaya, A.P.; Webb, S.; Martin, D.; Hajishengallis, G.; Divaris, K.; et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell 2021, 184, 4090–4104.
- Wang, M.; Krauss, J.L.; Domon, H.; Hosur, K.B.; Liang, S.; Magotti, P.; Triantafilou, M.; Triantafilou, K.; Lambris, J.D.; Hajishengallis, G. Microbial Hijacking of Complement–Toll-like Receptor Crosstalk. Sci. Signal. 2010, 3, ra11.
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyronomas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768–778.
- Hajishengallis, G.; Darveau, R.; Curtis, M. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725.
- Hajishengallis, G.; Lamont, R.; Graves, D. The enduring importance of animal models in understanding periodontal disease. Virulence 2015, 6, 229–235.
- Hajishengallis, G.; Lamont, R. The polymicrobial synergy and dysbiosis model of periodontal disease pathogenesis. In The Human Microbiota and Chronic Disease: Dysbiosis as a Cause of Human Pathology; John Wiley & Sons Inc.: New York, NY, USA, 2016; pp. 227–242.
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017.
- Abusleme, L.; Dupuy, A.K.; Nicol, D.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025.
- Yost, S.; Duran-Pinedo, A.E.; Teles, R.; Krishnan, K.; Frias-Lopez, J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 2015, 7, 27.
- Wang, G.P. Defining functional signatures of dysbiosis in periodontitis progression. Genome Med. 2015, 7, 4–6.
- Hirano, T.; Beck, D.A.C.; Demuth, D.R.; Hackett, M.; Lamont, R.J. Deep sequencing of Porphyronomas gingivalis and comparative transcriptome analysis of a LuxS mutant. Front. Cell. Infect. Microbiol. 2012, 2, 79.
- Frias-Lopez, J.; Duran-Pinedo, A. Effect of periodontal pathogens on the metatranscriptome of a healthy multispecies biofilm model. J. Bacteriol. 2012, 194, 2082–2095.
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54.
- Kimura, S.; Nagai, A.; Onitsuka, T.; Koga, T.; Fujiwara, T.; Hamada, S. Induction of experimental periodontitis in mice with Porphyronomas gingivalis-adhered ligatures. J. Periodontol. 2000, 71, 1167–1173.
- Lin, J.; Bi, L.; Yu, X.; Kawai, T.; Taubman, M.A.; Shen, B.; Han, X. Porphyronomas gingivalis exacerbates ligature-induced, RANKLdependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect. Immun. 2014, 82, 4127–4134.
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828.
- Sato, K.; Takahashi, N.; Kato, T.; Matsuda, Y.; Yokoji, M.; Yamada, M.; Nakajima, T.; Kondo, N.; Endo, N.; Yamamoto, R.; et al. Aggravation of collagen-induced arthritis by orally administered Porphyronomas gingivalis through modulation of the gut microbiota and gut immune system. Sci. Rep. 2017, 7, 6955.
- Kato, T.; Yamazaki, K.; Nakajima, M.; Date, Y.; Kikuchi, J.; Hase, K.; Ohno, H.; Yamazaki, K. Oral Administration of Porphyronomas gingivalis Alters the Gut Microbiome and Serum Metabolome. mSphere 2018, 3, e00460-18.
- Zhu, H.; Bilgin, M.; Snyder, M. Proteomics. Annu. Rev. Biochem. 2003, 72, 783–812.
- Kuboniwa, M.; Hendrickson, E.L.; Xia, Q.; Wang, T.; Xie, H.; Hackett, M.; Lamont, R.J. Proteomics of Porphyronomas gingivalis within a model oral microbial community. BMC Microbiol. 2009, 9, 98.
- Maeda, K.; Nagata, H.; Ojima, M.; Amano, A. Proteomic and transcriptional analysis of interaction between oral microbiota Porphyronomas gingivalis and Streptococcus oralis. J. Proteome Res. 2015, 14, 82–94.
- Stobernack, T.; Glasner, C.; Junker, S.; Gabarrini, G.; de Smit, M.; de Jong, A.; Otto, A.; Becher, D.; van Winkelhoff, A.J.; van Dijl, J.M. The extracellular Proteome and Citrullinome of the Oral Pathogen Porphyronomas gingivalis. J. Proteome Res. 2016, 15, 4532–4543.
- Veith, P.D.; Shoji, M.; Scott, N.E.; Reynolds, E.C. Characterization of the O-Glycoproteome of Porphyronomas gingivalis. Microbiol. Spectr. 2022, 10, e01502-21.
- Veith, P.D.; Chen, Y.-Y.; Gorasia, D.G.; Chen, D.; Glew, M.D.; O’Brien-Simpson, N.M.; Cecil, J.D.; Holden, J.A.; Reynolds, E.C. Porphyronomas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 2014, 13, 2420–2432.
- Veith, P.D.; Luong, C.; Tan, K.H.; Dashper, S.G.; Reynolds, E.C. Outer membrane vesicle proteome of Porphyronomas gingivalis Is Differentially Modulated Relative to the Outer Membrane in Response to Heme Availability. J. Proteome Res. 2018, 17, 2377–2389.
- Gawron, K.; Wojtowicz, W.; Łazarz-Bartyzel, K.; Łamasz, A.; Qasem, B.; Mydel, P.; Chomyszyn-Gajewska, M.; Potempa, J.; Mlynarz, P. Metabolomic status of the oral cavity in chronic periodontitis. Vivo 2019, 33, 1165–1174.
- Su, W.; Shi, J.; Zhao, Y.; Yan, F.; Lei, L.; Li, H. Porphyronomas gingivalis triggers inflammatory responses in periodontal ligament cells by succinate-succinate dehydrogenase–HIF–1α axis. Biochem. Biophys. Res. Commun. 2020, 522, 184–190.
- Dong, Z.; Lv, W.; Zhang, C.; Chen, S. Correlation analysis of gut microbiota and serum metabolome with Porphyronomas gingivalis-induced metabolic disorders. Front. Cell. Infect. Microbiol. 2022, 12, 858902.
- Payne, J.B.; Golub, L.M.; Thiele, G.M.; Mikuls, T.R. The link between Periodontitis and Rheumatoid Arthritis: A Periodontist’s Perspective. Curr. Oral Health Rep. 2015, 2, 20–29.
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Mol. Cell Biol. 2016, 17, 451–459.
