Endodontics is an important sub-branch of dentistry which deals with the different conditions of pulp to prevent tooth loss. Traditionally, common procedures, namely pulp capping, root canal treatment, apexification, and apexigonesis, have been considered for the treatment of different pulp conditions using selected materials. However, clinically to regenerate dental pulp, tissue engineering has been advocated as a feasible approach. New trends are emerging in terms of regenerative endodontics which have led to the replacement of diseased and non-vital teeth into the functional and healthy dentine-pulp complex. Root- canal therapy is the standard management option when dental pulp is damaged irreversibly. This treatment modality involves soft-tissue removal and then filling that gap through the obturation technique with a synthetic material. The formation of tubular dentine and pulp-like tissue formation occurs when stem cells are transplanted into the root canal with an appropriate scaffold material. To sum up tissue engineering approach includes three components: (1) scaffold, (2) differentiation, growth, and factors, and (3) the recruitment of stem cells within the pulp or from the periapical region.
- endodontia
- regenerative endodontics
- revascularization
1. Introduction
2. Development of Regenerative Endodontic Procedures (REP)
3. Revascularization or Revitalization
3.1. Advantages of the Revascularization Approach
-
Technically simple approach.
-
There is no need of using expensive biotechnology due to currently available instruments and medicament techniques.
-
There are almost negligible chances of immune rejection as this approach relies on the patient’s own blood.
-
Bacterial microleakage can be eliminated through the induction of stem cells into the root canal space, followed by the intra-canal barrier, inducing a blood clot.
-
The concerns of restoration retention need to be overcome.
-
When this approach is applied to immature teeth, it reinforces their root walls.
-
As the avulsed immature tooth has necrotic-pulp tissue along with an open apex, and short and intact roots; therefore, the newly formed tissue will easily reach the coronal-pulp horn because proliferation in a short distance is required. Therefore, the strategy behind the development of new tissue is to maintain the balance between the pulp-space infection and the proliferation of new tissue.
-
Additional growth of open-apex root takes place due to minimum instrumentation that will preserve viable pulp tissue.
-
The potential to regenerate more stem cells and the rapid capacity to heal the tissue in young patients needs to be recognised.
3.2. Disadvantages of the Revascularization Approach
-
The origin of where the tissue has been regenerated from is yet to be known.
-
According to researchers, effective composition and concentration of cells are mandatory for tissue engineering. However, these cells are entombed in fibrin clots; therefore, researchers do not rely on blood-clot formation for tissue engineering function.
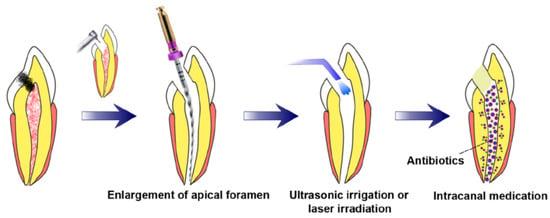
3.3. Prerequisites for Revascularization Approach (Figure 1)
-
There should be open apices and necrotic pulp secondary to trauma.
-
In addition, open apex should be less than 1.5 mm.
-
The following agents can be incorporated to remove microorganisms from the canal.
-
The coronal seal should be effective.
-
There should be a matrix or the growth of new tissues.
-
When trying to induce bleeding, anaesthesia should be used without a vasoconstrictor [70].
-
Canals should not be instrumented.
-
Sodium hypochlorite should be used as the irrigant.
-
There should be blood-clot formation.
4. Postnatal Stem Cell Therapy
Bone, buccal mucosa, fat, and skin are the common sources of postnatal-stem cells. After the apex is opened, the disinfected root-canal system is injected with postnatal-stem cells. This treatment is considered the simplest technique [72]. There are numerous benefits of this type of tissue-engineering technique. Postnatal-stem cells are rationally easy to harvest, and these cells can persuade the regeneration of the pulp. Moreover, these cells are easy to deliver by syringe. In addition, application of these stem-cell therapy is used in regenerative medicine since past many years, for example, bone-marrow replacement and endodontic applications [73]. However, low survival rates are one of the major disadvantages of this technique. Moreover, these cells can migrate into different locations of the body, which presents peculiar forms of mineralization [74]. For the development of dental tissues by the differentiation of stem cells, bioactive-signalling molecules, growth factors, and scaffolds are required [75]. Consequently, with only stem cells that exclude the growth factors or scaffolds, the chance of pulpal regeneration of new tissues is very low. In this approach, the chief identification of a postnatal-stem-cell source that must be able to differentiate into the diverse cell population can be obtained [74]. However, this technique is not approved yet.4.1. Pulp Implantation
4.2. Scaffold Implantation
4.3. Three-Dimensional Cell Printing
4.4. Gene Therapy
4.5. Nitric Oxide
4.6. Platelet-Rich Plasma (PRP)
4.7. Cell Homing
In tissue regeneration, the first concept of cell homing was presented in Lancet in 2010. The concept was based on the delivery of transforming growth factor-b3 (TGFb3) without cell transplantation. This approach was first used for the regeneration of the articular cartilage [114]. However, for dental-tissue regeneration, the idea of cell homing was introduced in 2010 [115]. During cell homing, root canal of the extracted human teeth was shaped and cleaned followed by the delivery of the growth factors, scaffold, and stem cells. Residual proteins in the root canal or dentinal tubules were deactivated in the first phase. This can be done by sterilization of extracted teeth in an autoclave. This was followed by the infusion of collagen gel into a shaped and cleaned root canal that might be with or without basic fibroblast growth factors (bFGFs), vascular endothelial growth factors (VEGFs), platelet-derived growth factors (PDGFs), nerve growth factors (NGFs), or bone morphogenetic proteins (BMPs).
The prime difference between the cell homing and cell transplantation approaches is that, in the latter case, for dentine/pulp regeneration, the isolated cells (stem/progenitor) from the host are transplanted into the root canal of the host. Dental pulp-like cells have been differentiated in the cell-homing approach when growth factors are recruited into the root-canal system. Cell-homing-technique-dental-organ regeneration presents a harmonizing and/or balancing approach to cell-transplantation technique and, at the same time, this strategy has shown auspicious results in animal models [23,115,116]. Hematopoietic-stem cells were militarized and transferred to different tissues or organs using active navigation in the cell-homing approach. The ultimate outcome of this process is pulp-dentin re-cellularization and revascularization. Numerous growth factors along with cell homing will result in pulp-dentin regeneration. Tissue revascularization and regeneration-cell homing consist of two distinctive cellular processes. They are differentiation and recruitment [117,118].
5. Biomimetic Materials in Endodontics
5.1. Biointeractive Materials
5.1.1. Calcium Hydroxide
5.1.2. Calcium Sulfate
5.2. Bioactive Materials
“A bioactive material is one that elicits a specific biological response at the interface of the material which results in the formation of a bond between the tissues and the material” [151].
5.2.1. Calcium Silicate Based-Cements
Mineral Trioxide Aggregate
Biodentine
Calcium Aluminate Cement
Theracal
5.2.2. Calcium Phosphate Based Cements
Hydroxyapatite
Bioactive Glass
5.2.3. Mixture of Calcium Silicate and Phosphate Based-Cements
Bioaggregate
Endosequence Root Repair Material
5.2.4. Sealer
Endosequence BC Sealer
5.2.5. Gutta-Percha
Bioceramic Coated Gutta-Percha
5.3. Remineralizing Agents
5.3.1. Enamel Matrix Derivative (Emdogain) Remineralizing Agent
5.3.2. Dentine Matrix Derivative/Demineralized Dentin Matrix
5.4. Miscellaneous
Calcium Phosphate Cements
6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics7040229
