The Coronavirus disease 2019 (COVID-19) pandemic has negatively affected the treatment of malignant diseases and the development of new prodrugs worldwide. Patients with cancer are more susceptible to a higher risk of coronavirus infection and its severe complications than the general population. The lungs are the most strongly affected organs in SARS-CoV-2 infection. Additionally, within the lungs, as in other human organ tissues, angiotensin-converting enzyme 2 (ACE2) was proven to be the main host cell receptor for the binding of SARS-CoV-2. ACE2 expression is also elevated in tumor and tumor-adjacent normal tissues in patients with lung cancer, which might partially explain why patients with lung cancer are potentially at a higher risk of severe COVID-19.
- COVID-19
- vaccine
- lung cancer
- immune checkpoint inhibitors
1. COVID-19 and Lung Cancer
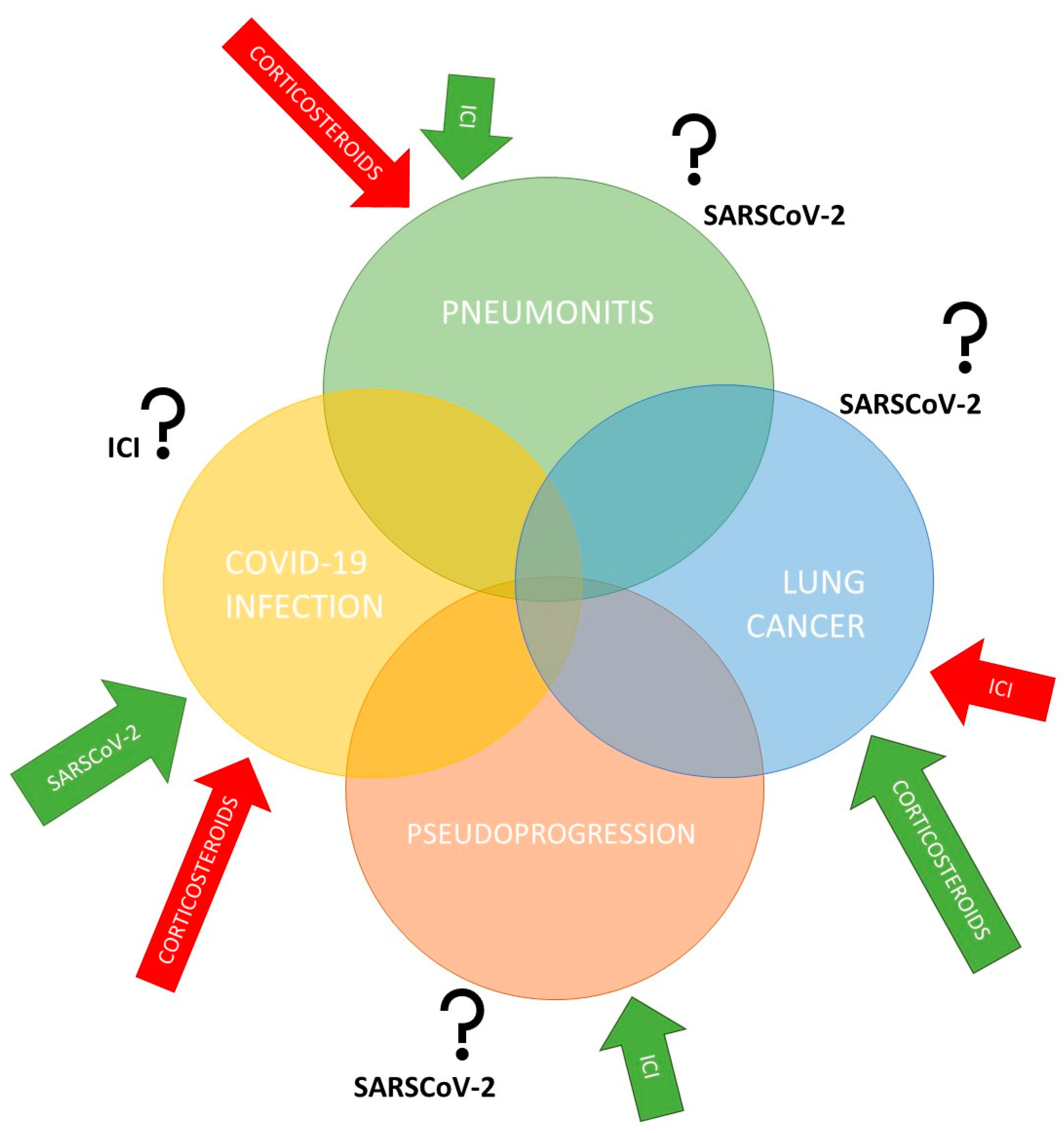
2. COVID-19 and Anticancer Treatment with Immune Checkpoint Inhibitors
3. COVID-19 Vaccines in Patients with Cancer
Studies on more than 1000 cancer patients have investigated the COVID-19 vaccine’s safety and effectiveness, including the early safety profile of the BNT162b2 vaccine in 134 patients with cancer (including lung cancer) under immune checkpoint blockage [34][35][36][37][38][39][40]. The most common side effects were mild and similar to those of other common vaccines [41]. Initial data suggest that SARS-CoV-2 vaccines are effective in patients with cancer. However, most studies only assessed seroconversion in patients with cancer, and only some studies performed neutralization assays to measure neutralizing antibody responses against variants of concern [42][43].
Moreover, patients with solid and hematologic cancer were not included in pivotal clinical trials conducted to demonstrate the efficacy and safety of COVID-19 vaccines. Patients with cancer appear to be more likely to develop a less efficient immune response following vaccination against COVID-19. Prior to the predominance of the Omicron variant, this ability was assessed to be lower than that in immunocompetent individuals [39][44][45][46][47][48][49][50].
Risk factors that can impair seroconversion in patients with solid tumors include older age, male sex, vaccine type, and chronic corticosteroid use. The influence of chemotherapy and immunotherapy on the efficacy of COVID-19 vaccines is of special interest. Recent chemotherapy has been repeatedly identified as a strong risk factor for lower seroconversion and neutralizing responses [42][51][52]. Oosting et al. recently reported the impact of immunotherapy, chemotherapy, and chemoimmunotherapy on the immunogenicity and safety of the COVID-19 vaccination in patients treated for solid tumors.
Data from studies involving patients with cancer so far indicate that the adverse events did not differ based on registration with the various vaccine platforms. The most common adverse events reported were soreness or pain at or around the injection site (63% of vaccines), local swelling (9%), muscle pain (34%), fatigue (34%), headache (16%), fever (10%), chills (10%), and gastrointestinal events (10%). Of course, there is still no knowledge of the long-term adverse effects of COVID-19 vaccines in individuals with and without cancer due to the very short observation time [40][43]. Patients receiving immunocheckpoint inhibitors were at possible additional risk of developing irAEs after the COVID-19 vaccination, but this was not confirmed [40]. Radiation phenomena, such as pneumonitis or dermatitis, have also been described following the COVID-19 vaccination and can sometimes significantly complicate discernment; awareness of this complication is important to avoid mistaking such phenomena as adverse effects of cancer therapy [53][54].
This entry is adapted from the peer-reviewed paper 10.3390/ijms232315067
References
- Aboueshia, M.; Hussein, M.H.; Attia, A.S.; Swinford, A.; Miller, P.; Omar, M.; Toraih, E.A.; Saba, N.; Safah, H.; Duchesne, J.; et al. Cancer and COVID-19: Analysis of Patient Outcomes. Future Oncol. 2021, 17, 3499–3510.
- Guan, W.; Liang, W.; Zhao, Y.; Liang, H.; Chen, Z.; Li, Y.; Liu, X.; Chen, R.; Tang, C.; Wang, T.; et al. Comorbidity and Its Impact on 1590 Patients with COVID-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020, 55, 2000547.
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer Patients in SARS-CoV-2 Infection: A Nationwide Analysis in China. Lancet Oncol. 2020, 21, 335–337.
- Yu, J.; Ouyang, W.; Chua, M.L.K.; Xie, C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020, 6, 1108.
- Desai, A.; Sachdeva, S.; Parekh, T.; Desai, R. COVID-19 and Cancer: Lessons From a Pooled Meta-Analysis. JCO Glob. Oncol. 2020, 6, 557–559.
- Moujaess, E.; Kourie, H.R.; Ghosn, M. Cancer Patients and Research during COVID-19 Pandemic: A Systematic Review of Current Evidence. Crit. Rev. Oncol./Hematol. 2020, 150, 102972.
- Qu, J.; Yang, R.; Song, L.; Kamel, I.R. Atypical Lung Feature on Chest CT in a Lung Adenocarcinoma Cancer Patient Infected with COVID-19. Ann. Oncol. 2020, 31, 825–826.
- Zhu, W.J.; Wang, J.; He, X.H.; Qin, Y.; Yang, S.; Hu, X.S.; Wang, H.Y.; Huang, J.; Zhou, A.P.; Ma, F.; et al. The differential diagnosis of pulmonary infiltrates in cancer patients during the outbreak of the 2019 novel coronavirus disease. Zhonghua Zhong Liu Za Zhi 2020, 42, 305–311.
- Jin, X.-H.; Zheng, K.I.; Pan, K.-H.; Xie, Y.-P.; Zheng, M.-H. COVID-19 in a Patient with Chronic Lymphocytic Leukaemia. Lancet Haematol. 2020, 7, e351–e352.
- Das, S.; Johnson, D.B. Immune-Related Adverse Events and Anti-Tumor Efficacy of Immune Checkpoint Inhibitors. J. ImmunoTher. Cancer 2019, 7, 306.
- Quach, H.; Dewan, A.; Davis, E.; Ancell, K.; Fan, R.; Ye, F.; Johnson, D. Association of Anti–Programmed Cell Death 1 Cutaneous Toxic Effects With Outcomes in Patients With Advanced Melanoma. JAMA Oncol. 2019, 5, 906–908.
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527.
- Gorospe, L.; Ayala-Carbonero, A.M.; Paredes-Rodríguez, P.; Muñoz-Molina, G.M.; Arrieta, P.; Mirambeaux-Villalona, R.M.; Vallejo-Ocaña, M.C.; Benito-Berlinches, A.; Lage-Alfranca, Y.; Gómez-Rueda, A. Challenges in Management of Patients With Lung Cancer in Times of COVID-19: An Imaging Perspective. Clin. Lung Cancer 2020, 21, 568–570.
- Zhang, Y.-J.; Yang, W.-J.; Liu, D.; Cao, Y.-Q.; Zheng, Y.-Y.; Han, Y.-C.; Jin, R.-S.; Han, Y.; Wang, X.-Y.; Pan, A.-S.; et al. COVID-19 and Early-Stage Lung Cancer Both Featuring Ground-Glass Opacities: A Propensity Score-Matched Study. Transl. Lung Cancer Res. 2020, 9, 1516–1527.
- Zhu, J.; Zhang, Y.; Gao, X.-H.; Xi, E.-P. Coronavirus Disease 2019 or Lung Cancer: A Differential Diagnostic Experience and Management Model From Wuhan. J. Thorac. Oncol. 2020, 15, e141–e142.
- Rogiers, A.; Pires da Silva, I.; Tentori, C.; Tondini, C.A.; Grimes, J.M.; Trager, M.H.; Nahm, S.; Zubiri, L.; Manos, M.; Bowling, P.; et al. Clinical Impact of COVID-19 on Patients with Cancer Treated with Immune Checkpoint Inhibition. J. Immunother. Cancer 2021, 9, e001931.
- Dobre, I.A.; Frank, A.J.; D’Silva, K.M.; Christiani, D.C.; Okin, D.; Sharma, A.; Montesi, S.B. Outcomes of Patients With Interstitial Lung Disease Receiving Programmed Cell Death 1 Inhibitors: A Retrospective Case Series. Clin. Lung Cancer 2021, 22, e738–e744.
- Sullivan, R.J.; Johnson, D.B.; Rini, B.I.; Neilan, T.G.; Lovly, C.M.; Moslehi, J.J.; Reynolds, K.L. COVID-19 and Immune Checkpoint Inhibitors: Initial Considerations. J. Immunother. Cancer 2020, 8, e000933.
- Johnson, D.B.; Taylor, K.B.; Cohen, J.V.; Ayoubi, N.; Haugh, A.M.; Wang, D.Y.; Schlick, B.D.; Voorhees, A.L.; Gage, K.L.; Fintelmann, F.J.; et al. Anti–PD-1–Induced Pneumonitis Is Associated with Persistent Imaging Abnormalities in Melanoma Patients. Cancer Immunol. Res. 2019, 7, 1755–1759.
- Rodrigues, R.; Costa de Oliveira, S. The Impact of Angiotensin-Converting Enzyme 2 (ACE2) Expression Levels in Patients with Comorbidities on COVID-19 Severity: A Comprehensive Review. Microorganisms 2021, 9, 1692.
- Winkler, T.; Ben-David, U. Elevated Expression of ACE2 in Tumor-Adjacent Normal Tissues of Cancer Patients. Int. J. Cancer 2020, 147, 3264–3266.
- Liu, A.; Zhang, X.; Li, R.; Zheng, M.; Yang, S.; Dai, L.; Wu, A.; Hu, C.; Huang, Y.; Xie, M.; et al. Overexpression of the SARS-CoV-2 Receptor ACE2 Is Induced by Cigarette Smoke in Bronchial and Alveolar Epithelia. J. Pathol. 2021, 253, 17–30.
- Jia, W.; Wang, J.; Sun, B.; Zhou, J.; Shi, Y.; Zhou, Z. The Mechanisms and Animal Models of SARS-CoV-2 Infection. Front. Cell Dev. Biol. 2021, 9, 1129.
- Howells, A.; Marelli, G.; Lemoine, N.R.; Wang, Y. Oncolytic Viruses—Interaction of Virus and Tumor Cells in the Battle to Eliminate Cancer. Front. Oncol. 2017, 7, 195.
- Shang, C.; Liu, Z.; Zhu, Y.; Lu, J.; Ge, C.; Zhang, C.; Li, N.; Jin, N.; Li, Y.; Tian, M.; et al. SARS-CoV-2 Causes Mitochondrial Dysfunction and Mitophagy Impairment. Front. Microbiol. 2022, 12, 780768.
- Taghizadeh-Hesary, F.; Akbari, H.; Bahadori, M.; Behnam, B. Targeted Anti-Mitochondrial Therapy: The Future of Oncology. Genes 2022, 13, 1728.
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019, 5, 1411–1420.
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355.
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Bersanelli, M. Controversies about COVID-19 and Anticancer Treatment with Immune Checkpoint Inhibitors. Immunotherapy 2020, 12, 269–273.
- Grivas, P.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.-Y.; Shyr, Y.; Li, X.; Choueiri, T.K.; Painter, C.A.; et al. Association of Clinical Factors and Recent Anticancer Therapy with COVID-19 Severity among Patients with Cancer: A Report from the COVID-19 and Cancer Consortium. Ann. Oncol. 2021, 32, 787–800.
- Chen, C.; Zhang, X.R.; Ju, Z.Y.; He, W.F. Advances in the research of mechanism and related immunotherapy on the cytokine storm induced by coronavirus disease 2019. Zhonghua Shao Shang Za Zhi 2020, 36, 471–475.
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422.
- Barrière, J.; Re, D.; Peyrade, F.; Carles, M. Current Perspectives for SARS-CoV-2 Vaccination Efficacy Improvement in Patients with Active Treatment against Cancer. Eur. J. Cancer 2021, 154, 66–72.
- Goshen-Lago, T.; Waldhorn, I.; Holland, R.; Szwarcwort-Cohen, M.; Reiner-Benaim, A.; Shachor-Meyouhas, Y.; Hussein, K.; Fahoum, L.; Baruch, M.; Peer, A.; et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1507–1513.
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140.
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion Rates Following COVID-19 Vaccination among Patients with Cancer. Cancer Cell 2021, 39, 1081–1090.e2.
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.-Y.; Taylor, B.S.; Simand, P.-F.; et al. Immunogenicity of SARS-CoV-2 Messenger RNA Vaccines in Patients with Cancer. Cancer Cell 2021, 39, 1091–1098.e2.
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; Del Barrio, I.D.M.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and Immunogenicity of One versus Two Doses of the COVID-19 Vaccine BNT162b2 for Patients with Cancer: Interim Analysis of a Prospective Observational Study. Lancet Oncol. 2021, 22, 765–778.
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-Term Safety of the BNT162b2 MRNA COVID-19 Vaccine in Patients with Cancer Treated with Immune Checkpoint Inhibitors. Lancet Oncol. 2021, 22, 581–583.
- Rossi, G.; Pezzuto, A.; Sini, C.; Tuzi, A.; Citarella, F.; McCusker, M.G.; Nigro, O.; Tanda, E.; Russo, A. Concomitant Medications during Immune Checkpoint Blockage in Cancer Patients: Novel Insights in This Emerging Clinical Scenario. Crit. Rev. Oncol. Hematol. 2019, 142, 26–34.
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive Immunity and Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Following Vaccination in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1305–1320.
- Oosting, S.F.; van der Veldt, A.A.M.; GeurtsvanKessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.-M.C.; Smit, E.F.; Hiltermann, T.J.N.; den Hartog, G.; Jalving, M.; et al. MRNA-1273 COVID-19 Vaccination in Patients Receiving Chemotherapy, Immunotherapy, or Chemoimmunotherapy for Solid Tumours: A Prospective, Multicentre, Non-Inferiority Trial. Lancet Oncol. 2021, 22, 1681–1691.
- Embi, P.J. Effectiveness of 2-Dose Vaccination with MRNA COVID-19 Vaccines Against COVID-19–Associated Hospitalizations Among Immunocompromised Adults—Nine States, January–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1553–1559.
- Linardou, H.; Spanakis, N.; Koliou, G.-A.; Christopoulou, A.; Karageorgopoulou, S.; Alevra, N.; Vagionas, A.; Tsoukalas, N.; Sgourou, S.; Fountzilas, E.; et al. Responses to SARS-CoV-2 Vaccination in Patients with Cancer (ReCOVer Study): A Prospective Cohort Study of the Hellenic Cooperative Oncology Group. Cancers 2021, 13, 4621.
- Shmueli, E.S.; Itay, A.; Margalit, O.; Berger, R.; Halperin, S.; Jurkowicz, M.; Levin, E.G.; Levy, I.; Olmer, L.; Regev-Yochay, G.; et al. Efficacy and Safety of BNT162b2 Vaccination in Patients with Solid Cancer Receiving Anticancer Therapy—A Single Centre Prospective Study. Eur. J. Cancer 2021, 157, 124–131.
- Ligumsky, H.; Safadi, E.; Etan, T.; Vaknin, N.; Waller, M.; Croll, A.; Nikolaevski-Berlin, A.; Greenberg, I.; Halperin, T.; Wasserman, A.; et al. Immunogenicity and Safety of the BNT162b2 MRNA COVID-19 Vaccine Among Actively Treated Cancer Patients. JNCI J. Natl. Cancer Inst. 2022, 114, 203–209.
- Barrière, J.; Chamorey, E.; Adjtoutah, Z.; Castelnau, O.; Mahamat, A.; Marco, S.; Petit, E.; Leysalle, A.; Raimondi, V.; Carles, M. Impaired Immunogenicity of BNT162b2 Anti-SARS-CoV-2 Vaccine in Patients Treated for Solid Tumors. Ann. Oncol. 2021, 32, 1053–1055.
- Becerril-Gaitan, A.; Vaca-Cartagena, B.F.; Ferrigno, A.S.; Mesa-Chavez, F.; Barrientos-Gutiérrez, T.; Tagliamento, M.; Lambertini, M.; Villarreal-Garza, C. Immunogenicity and Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection after Coronavirus Disease 2019 (COVID-19) Vaccination in Patients with Cancer: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2022, 160, 243–260.
- Mair, M.J.; Berger, J.M.; Berghoff, A.S.; Starzer, A.M.; Ortmayr, G.; Puhr, H.C.; Steindl, A.; Perkmann, T.; Haslacher, H.; Strassl, R.; et al. Humoral Immune Response in Hematooncological Patients and Health Care Workers Who Received SARS-CoV-2 Vaccinations. JAMA Oncol. 2022, 8, 106–113.
- Peeters, M.; Verbruggen, L.; Teuwen, L.; Vanhoutte, G.; Vande Kerckhove, S.; Peeters, B.; Raats, S.; Van der Massen, I.; De Keersmaecker, S.; Debie, Y.; et al. Reduced Humoral Immune Response after BNT162b2 Coronavirus Disease 2019 Messenger RNA Vaccination in Cancer Patients under Antineoplastic Treatment. ESMO Open 2021, 6, 100274.
- Rousseau, B.; Loulergue, P.; Mir, O.; Krivine, A.; Kotti, S.; Viel, E.; Simon, T.; de Gramont, A.; Goldwasser, F.; Launay, O.; et al. Immunogenicity and Safety of the Influenza A H1N1v 2009 Vaccine in Cancer Patients Treated with Cytotoxic Chemotherapy and/or Targeted Therapy: The VACANCE Study. Ann. Oncol. 2012, 23, 450–457.
- Soyfer, V.; Gutfeld, O.; Shamai, S.; Schlocker, A.; Merimsky, O. COVID-19 Vaccine-Induced Radiation Recall Phenomenon. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 957–961.
- Stewart, R.; McDowell, L. Radiation Recall Phenomenon Following COVID-19 Vaccination. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 835–836.
