Dendritic cells (DCs) are unique cellular drivers orchestrating adaptive immune response. In the light of the recent success of immune checkpoint inhibitors (ICI) in cancer treatment, DC have recently been reconsidered as critical cellular adjuvants in cancer vaccination. In fact, increasing the presentation of tumor antigens remains one of the major issues for eliciting a strong antitumor immune response. A number of studies have confirmed that type I IFN can efficiently promote the differentiation of peripheral blood monocytes into potent DC (IFN-DC), orientating DC functions towards the priming and expansion of protective antitumor immune responses. IFN-DC can be conveniently exploited for the design of effective strategies of cancer vaccination as a monotherapy or in combination with immune-checkpoint inhibitors or immunomodulatory drugs.
- Type I IFN
- dendritic cells
- therapeutic vaccines
- Cancer
1. Introduction
Cancer immunotherapy is typically aimed at stimulating or enhancing antitumor immune response in oncological patients. Among different immunotherapeutic approaches, therapeutic cancer vaccines are designed to instruct the immune system to identify and eradicate tumor cells, while preserving normal cells and tissues from immune attack, presumably preventing undesirable side effects. Cancer vaccines have the potential to control tumors as monotherapy or in combination with other forms of immunotherapy, as well as with nonimmune-based therapies, such as radiotherapy or chemotherapy. In particular, in patients with minimal residual disease after tumor debulking, this therapeutic option may result in prolonged survival and improved life quality. As a consequence of the recent success of immune checkpoint inhibitors (ICI) in the treatment of cancer patients [1], dendritic cells (DC), specialized in sensitizing lymphocytes to tumor antigens, have gained renewed interest as critical cell adjuvants in immunotherapeutic approaches. In particular, DC-based vaccines and T-cell checkpoint blockade can act as synergistic partners, as checkpoint inhibitors simply function as boosters of immune responses and their efficacy is proportional to the pre-existing amount of tumor-specific T cells at the tumor site.
2. The Link between Type I IFN and DC in Cancer Rejection
DC are professional antigen presenting cells (APC), acting at the interface between the environment and the immune system and bridging the gap between innate and adaptive immunity [2]. By virtue of their unique ability to take up and process antigens in the peripheral blood and tissues, DC play a crucial role in the initiation of primary immune responses. Upon maturation/activation, DC undergo phenotypic changes, increase MHC and costimulatory molecule expression, and upregulate cytokine production. Mature DC promptly migrate to draining lymph nodes, to prime naïve T cells and initiate adaptive immune response [2]. Since their discovery, it has been shown that DC lineage is complex and includes a variety of different subsets: conventional DC (cDC), plasmacytoid DC (pDC), Langerhans cells and monocyte-derived DC (moDC). DC have attracted considerable attention as potential cell-drugs in the preparation of therapeutic cancer vaccines. Cancer vaccination has been performed using reinfusion of defined populations of DC obtained ex vivo from peripheral blood, including the use of BDCA1+ cDC and pDC [3,4,5]. However, the scarceness of these DC subsets in the peripheral blood has so far imposed major limitations to their use in the clinical setting. Therefore, most DC-based vaccines have exploited moDC differentiated ex vivo from monocytes cultured in the presence of IL-4 and GM-CSF or other cytokines, because of the relative ease of recovering large numbers of these cells from the peripheral blood. However, the choice of an optimal protocol of DC generation in vitro for the preparation of clinically effective therapeutic cancer vaccines still represents a major challenge. While the optimal culture conditions for generating the most effective moDC is still controversial, some groups, including ours, have shown that partially mature and highly active moDC from blood monocytes can be rapidly generated in the presence of IFN-α and GM-CSF (IFN-DC) [6,7].
Although type I IFN (IFN-α and IFN-β; hereafter IFN-I) was originally characterized for its antiviral activity [8], it is also known to mediate antiproliferative and antineoplastic effects and proved the most useful and wide-ranging biologic agent against several tumors [9]. IFN-α has been used for the treatment of selected tumors, including melanoma and renal cancer, showing its best efficacy in hematological malignancies, such as hairy cell leukemia, chronic myeloid leukemia, and follicular lymphoma [10]. Direct evidence of IFN-α activity in both B and T-cell low-grade lymphomas is the regression of cutaneous and conjunctival neoplastic lesions following repeated in situ injections of this pleiotropic drug [11,12]. In solid tumors the results have been more disappointing. However, evidence exists showing that IFN-α can be beneficial against early stage cancers, but much less effective against established or metastatic tumors [13]. Due to adverse effects of systemic high dose IFN-α administration in cancer patients and the development of more effective drugs and protocols, the initial interest in IFN-based therapies rapidly faded down. Conversely, a growing interest has emerged on the immunomodulatory role of type I IFN, since a considerable body of evidence clearly demonstrates that IFN-α can bridge innate and adaptive immunity through its effects on DC differentiation/activation, skewing DC functions towards the priming and expansion of protective antitumor immune responses [14,15]. Studies to evaluate the direct effect of IFN-α on experimental and conventional vaccines in mice and humans have also been performed [16]. Nevertheless, a few pilot studies have also attempted to evaluate the possible immune modulating activity of these cytokines in vaccination strategies. In some of them, IFN-α induced improved immunological responses [17,18,19] or enhanced peptide immunogenicity [20,21,22]. On the whole, these findings are strongly consistent with studies performed over the last 20 years, showing the importance of IFN-α driven generation of highly active DC and the induction of adaptive immunity. Le Bon et al. demonstrated that DC were the cell type mediating the adjuvant effect of IFN-I in vivo, inducing long-term antibody production and immunological memory against a poorly immunogenic antigen [23]. In addition, DC activation by type I IFN can promote spontaneous immune responses to tumor cells, including the cross-priming of tumor-specific CD8 T cells, [24,25,26,27]. Therefore, the immune response for cancer rejection appears to exploit inflammatory mechanisms reminiscent of those activated in early antiviral defense mediated by type I IFN release from pDC and macrophages [28]. In this view, IFN-α may be involved in a proinflammatory condition promoting the in vivo conversion of monocytes into DC, initiating antiviral and antitumor specific immune responses. In fact, significant amounts of type I IFN can be locally released at the site of infection or inflammation. This may enable the differentiation of circulating monocytes into activated DC mediating the activation of natural killer cells, the generation of a Th1-polarized T-helper response, and the induction of a cytolytic response against both viruses and cancer cells. Worth mentioning, infiltrating IFN-DC have been demonstrated in regressing molluscum contagiosum skin lesions, characterized by the accumulation of pDC and the local production of type I IFN [29]. Reasonably, the culture conditions developed for the generation of IFN-DC in vitro may reproduce the natural cytokine milieu enabling the rapid differentiation of DC from monocyte in vivo and could be considered a physiological pathway of monocyte conversion into DC.
3. IFN-α-Conditioned Dendritic Cells (IFN-DC)
IFN-α and IFN-β differently modulate DC activation/maturation, depending on the experimental model and culture conditions. Indeed, IFN-α has been shown to markedly enhance DC maturation [30,31,32]. Moreover, IFN-α can synergize with polyinosinic:polycytidylic acid (p-I:C) and the “classical” type-1-polarizing cytokine cocktail, allowing for serum-free generation of fully mature type-1-polarized DC (DC1) [33,34], providing DC with different chemoattractive properties [35]. However, in 1998 it became apparent that IFN-α in itself was capable of driving the differentiation of blood monocytes into DC [36]. Soon after, our group reported that a three day culture in the presence of IFN-α and GM-CSF can convert blood monocytes into fully functional and partially mature DC (IFN-DC), without the addition of maturation factors or further culture steps [6,7]. Since then, numerous studies have confirmed that type I IFN can efficiently induce the differentiation of blood monocytes into DC favoring Th1 biased response, huge production of IFN-γ, and the efficient expansion of CD8 effector T cells [37,38,39,40,41]. As a result of IFN-α transcriptional signature, IFN-DC exhibit distinct molecular and functional features, showing a more advanced maturation phenotype, as compared to conventional moDC obtained with IL-4 and GM-CSF, with the expression of higher levels of costimulatory molecules as well as variable amounts of the maturation marker CD83 [6,7]. They also display mixed features of natural killer (NK cells) and pDC with significant levels of CD123 [7,29,38].
IFN-DC can efficiently initiate an adaptive immune response by virtue of the high expression of some important molecules involved in antigen processing, migration, and localization in the lymph nodes [28,29]. IFN-DC are endowed with improved migratory response to chemokines and express very high levels of CCR5. They exhibit an enhanced response to its ligands CCL5, CCL3, CCL4 as well. A considerable fraction of IFN-DC also expresses integrin α4 and CCR7 [42].
Moreover, IFN-DC demonstrate an improved migratory response to CCL19 and express significant levels of CCL19 themselves, together with CCL18 and CXCL10 [42]. Of note, high levels of monocyte chemoattractant proteins (MCPs), CXCL2 and CXCL-3 confer IFN-DC the capability to efficiently mediate the recruitment of other innate effector cells as well as a Th1-skewed cytokine production [38,43]. Despite their advanced maturation state, IFN-DC retain an efficient phagocytic activity [7], promptly acquiring a fully mature phenotype upon interaction with peripheral blood lymphocytes (PBL) [44]. IFN-DC can take up apoptotic cells through the scavenger receptor lectin-like oxidized-LDL receptor-1 (LOX-1) and cross-present their antigens to CD8+ T cells. [45]. IFN-DC are also directly licensed for CD4-independent CD8+ T cell priming, targeting antigen onto class I molecules, cross-presenting very efficiently low amounts of soluble proteins to CD8+ T cells [46]. Both immature and mature IFN-DC express high amounts of immunoproteasome subunits (LMP2, LMP7, and MECL1) along with elevated levels of TAP1, TAP2, calnexin, calreticulin, tapasin, and HLA class I molecules [47,48]. This functional attitude of IFN-DC results in very efficient triggering of specific CD8 T lymphocytes, specific for a subdominant MHC-I-restricted viral epitope and MART-127–35 epitope [48]. Noteworthy, the improved capacity of IFN-DC to protect internalized proteins from early degradation and to efficiently route antigens toward the MHC-I processing pathway, allows a long-lasting cross-priming capacity [49]. This suggests the potential ability of IFN-DC to retain antigens for an extended period in lymph nodes after their uptake, allowing the encounter and recruitment of rare specific CD8+ T-cell precursors, with important implications for the development of DC-based therapeutic vaccines. Importantly, IFN-DC also drive priming of naïve CD4 T cells, resulting in a massive expansion of CXCR3+ IFN-γ-producing CD4 Th1 cells [50]. IFN-DC express high levels of Fas-L and TRAIL, performing a direct tumoricidal activity [6,38,39,44]. Likewise, an important role of transmembrane TNF-α as mediator of IFN-DC killer activity, which becomes defective in high grade glioma patients, has been recently described [51]. The direct cytotoxic activity of IFN-DC against tumor cells represents an important functional feature, since it may facilitate tumor antigen uptake, resulting in earlier and improved induction of antitumor immune response.
4. IFN-DC in cancer immunotherapy
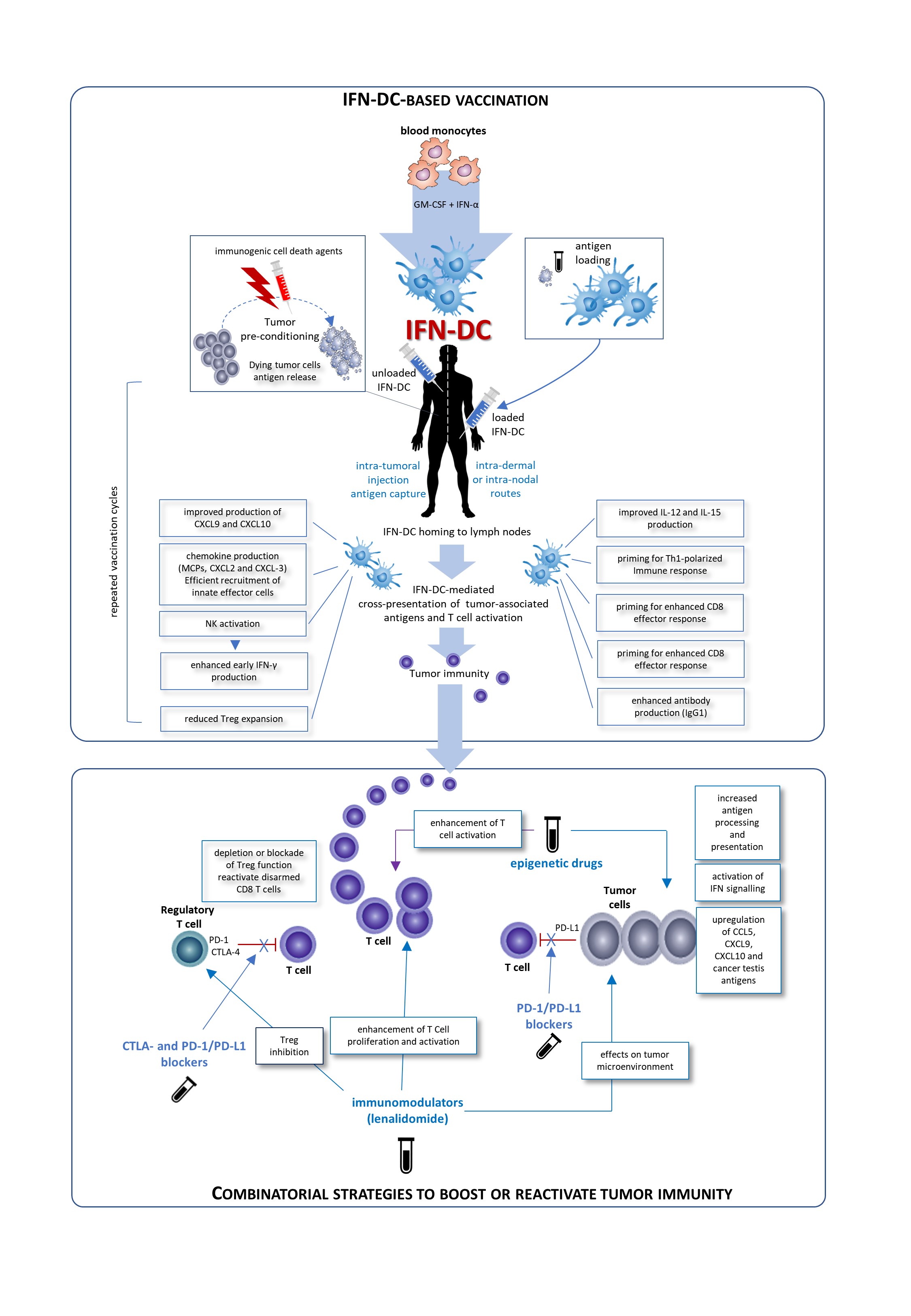
IFN-DC are a good candidate for a vaccinal clinical use in in cancer patients. Basically, two major modalities for the development of novel IFN-DC-based therapies can be envisaged: the standard administration of IFN-DC loaded with autologous tumor cells and intra-tumoral vaccination based on the concept of tumor pre-conditioning with immunogenic cell death agents followed by unloaded IFN-DC. Both approaches would finally culminate in the cross-presentation of tumor-associated antigens to CD8 T-cells and their activation. Importantly, IFN-DC loaded ex vivo or in vivo whole tumor cells offer the advantage of eliciting immunity against the entire collection of antigens expressed by the tumor, enabling a wider and more efficient anti-tumor immune response. In this regard, IFN-DC-vaccine based on whole tumor-cells induced to undergo immunogenic cell death can represent an optimal antigenic formulation for IFN-DC loading.
Fig 1. Possible clinical exploitation of IFN-DC in cancer vaccination. Patients undergo leukapheresis to collect PBMC and purify blood monocytes necessary for IFN-DC generation. Large numbers of partially-mature IFN-DC can be easily obtained at one-time-point from purified peripheral blood monocytes cultured in the presence IFN-α and GM-CSF, loaded or not with tumor antigens, and cryopreserved in ready-for-use aliquots for the programmed cycles of treatment. On the left is depicted the prototypical intra-tumoral vaccination strategy based on the concept of tumor pre-conditioning with immunogenic cell death agents followed by unloaded IFN-DC. The intra-tumoral injections are guided by ultrasound and performed by a radiologist to ensure correct administration. In the left right of the figure, the therapeutic vaccination strategy is shown. IFN are loaded in vitro with selected formulation of tumor antigens and administered intradermally, in close vicinity to axillary and inguinal lymph nodes or directly administered into a healthy lymph node. In both strategies the treatment cycles are repeated at two-week intervals. IFN-DC are characterized by the capacity to release a unique array of cytokines and chemokines known to favor Th1 type response and to powerfully stimulate cellular CD8+ T cell immune responses as well as promoting IgG1 isotype antibodies response [6].
Conceivably, IFN-DC-based monotherapy can evolve in chemotherapy-free combinatorial therapy regimens with immune-checkpoint inhibiting antibodies as well as immunomodulating or epigenetic drugs. The blockade of inhibitory pathways or activation promotes CD8 T cell priming after vaccination. Inhibition of Treg alleviates the suppressor activity of these cells on effector CD8+T cells. Blockade of the interaction of PD-1/PD-L1 reactivates disarmed CD8 cells and anti-tumor effector functions. An attractive immunomodulatory drug to be combined with IFN-DC-based therapies is lenalidomide, as it acts through the boosting of anti-tumor immunity and the modification of tumor microenvironment. Also epigenetic therapies for cancer including DNA methyltransferase inhibitors (DNMTi), histone deacetylase inhibitors (HDACi), and histone methyltransferase inhibitors (HMTi) can stimulate anti-tumor immunity in both tumor cells and host immune effector cells.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines8040617