Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Autoimmunity and cancer rates have both been on the rise in Western civilization leading many to question if there is a link between the two entities. Likewise, the association between immunodeficiency or induced immunodeficiency and malignancy has been at the forefront of medical research with particular interest in the transplanted patients, HIV patients and patients with autoimmune disease requiring chronic immunosuppression.
- autoimmunity
- immunodeficiency
- immunosurveillance
1. Introduction
The concept of an immune system whereby innate cells identify and destroy foreign or malfunctioning cells has intrigued scientists for decades. As knowledge expanded so did the complexity of investigations. In the 1950s–1970s, researchers Burnet and Thomas formulated the concept of cancer immunosurveillance whereby a functioning immune system, at the time thought to be cells derived from the Thymus, can recognize ‘transformed’ cells or tumor cells [1][2]. However, there was limited proof of this concept in animal experimental models several decades after the initial theory was published [3][4][5]. More recently this concept of the protective immunosurveillance has been redefined into a broad topic of cancer immunoediting with 3 distinct phases termed elimination, equilibrium and escape [6] The phases covered variety of functions including both the innate and adaptive immunity as well as tumor recognition and tumor modification [6]. Additional support of this concept is demonstrated by the discovery of mice without properly functioning lymphocytes developed malignancy at a significantly higher rate compared to the wild type [7]. Thus, properly functioning immune systems are beneficial in the targeting and destruction of tumor cells.
2. Autoimmunity and Cancer Correlation
Several studies have highlighted the connection between chronic inflammation and the development of malignancy [8]. While the exact mechanism of this relationship is not known, some speculate chronic inflammation leading to antigen specific cell damage, activation of Type 2 immune response mediated by IL-4 and IL-13, and relative CTLA-4 deficiency are potential triggers [8]. Other studies stipulate inflammatory cells themselves are a potential mechanism such as tumor-associated macrophages which can stimulate tumor growth and angiogenesis [9]. Observational representation of this concept can be seen in the increase in rates of autoimmune gastritis and its associated increase in rates of gastric cancer in younger women who have the highest incidence of autoimmune disease in comparison with a previously high prevalence of gastric cancer in men [10].
The association between autoimmunity induced inflammation and malignancy does not have a negative outcome always. A review by Zityogel et al. discussed the concept of ‘beneficial autoimmunity’ and identified examples of therapy-induced (such as immune checkpoint inhibitors) as well as idiopathic or spontaneous autoimmune disease conferring favorable outcomes against oncologic disease [11]. A higher level of T cells that recognize tumor-specific antigens available for anticancer immunosurveillance is thought to be another mechanism of beneficial autoimmunity [11]. In a large SEER [Surveillance, Epidemiology, and End Results program] database study, 13.5% of lung cancer patients were diagnosed with autoimmune disease during or after their cancer diagnosis [12]. However, a retrospective cohort study comparing lung cancer patients with and without autoimmune disease did not find any significant difference in progression-free survival per stage among the two groups, even though the autoimmune group was less likely to receive the standard of care inferring some protective benefit from the autoimmune disease [13]. In paraneoplastic encephalomyelitis, most commonly seen in patients with small cell lung cancer [SCLC], there was a favorable response to chemotherapy observed in patients who tested positive for anti-hu antibodies, autoantibodies against neuronal RNA binding proteins [14][15].
Given the evidence of beneficial autoimmunity, it would appear immunosuppression is a larger contributor to malignancy risk compared to autoimmune disease itself. Further evidence demonstrated in a 2020 review on celiac’s disease [treated with dietary modification] which found that overall malignancy risk was low and even negligible one year after diagnosis [16]. Likewise, a European study of autoimmune thyroiditis treated with hormone therapy did not find an elevated risk of thyroid cancer after a 10-year follow up period [17][18]. On the other hand, a Taiwanese study did find an increased risk of both thyroid cancer and colorectal cancer among Hashimoto’s thyroiditis patient population [19]. From this mixed data autoimmune disease must have a role in the development of malignancy.
Interestingly, malignancy has also been shown to stimulate autoimmunity, most notably through well-known paraneoplastic syndromes [20]. Paraneoplastic rheumatologic conditions such paraneoplastic polyarthritis and hypertrophic osteoarthropathy are a few notable examples and are thought be related to upregulation of VEGF and fibroblast growth factor 23 (FGF23) in tumors [20]. SCLC is another typical example of malignancy and associated with a high frequency of paraneoplastic syndromes that can proceed the diagnosis or be the first sign of relapse [21].
3. Immunodeficiency and Cancer Correlation
While overactive immune systems are a risk for malignancy, studies have also demonstrated that immunodeficiency may also be an independent risk factor [22]. This concept was first examined in mice models. In a research study, mice without an innate immune system were more vulnerable to both spontaneous and externally induced cancers [23]. A study involving the US Immune Deficiency Network Database examine primary immunodeficiency patients which included patients from 39 academic medical centers and >300 single gene mutations of the immune system in comparison to the age-adjusted SEER population. They identified a 1.42-fold increase in relative risk of malignancy in the primary immunodeficient population [22]. Interestingly, in subgroup analysis, men with primary immunodeficiency had the highest increase in relative risk, 1.91, compared to the age-adjusted population, while women were diagnosed with malignancy at similar rates [22].
Additional data provided by a large meta-analysis of HIV/AIDS patients [7 studies] and transplanted patients [5 studies] showed immunodeficiency and immunosuppression conferred a higher risk for malignancy in 20 out of the 28 types of cancer [24]. The authors proposed several mechanisms for the increased risk of malignancy including increased infections, immunodeficiency, increased cancer screening, and lifestyle factors [24]. However, the latter two theories were thought to be less likely as rates of screened cancers, breast and colon, were not elevated in both study populations and lifestyle factors were not standard across the study populations. Infection was thought to be an independent factor as many of the cancers in the analysis had an infectious cause [i.e., pylori, hepatitis, EBV] and several AIDS-defining malignancies were included [24]. However, notable exceptions were non-melanoma skin cancer and lip cancer that have no known infectious etiology and were found to have higher rates of malignancy in transplant recipients. Interestingly, the common epithelial-derived cancers, colon, breast, rectum, and ovarian, did not have higher rates among the immunosuppressed population [24].
4. Immunosuppression in Autoimmune Disease and Cancer Correlation
Autoimmune disease can be organ specific or systemic, but treatment and presentation vary greatly within each category. The mainstay of treatment in autoimmune disease can be separated into two categories, replacement therapy vs. immunosuppressive [25]. A NEJM review proposed that immunosuppressive treatment can be further characterized into 4 subtypes: alteration of thresholds of immune activation, modulation of antigen specific cells, reconstitution of the immune system and sparing of target organs [26]. Reconstitution of the immune system involves bone marrow ablation with or without the addition of stem cells, reserved for severe refractory disease as is an aggressive treatment [27]. More commonly used treatments target inflammatory pathways [26]. One such example are inhibitors of the TNF receptor as binding of its substrate triggers downstream signaling leading to upregulation of inflammation and apoptosis (Figure 1) [28].
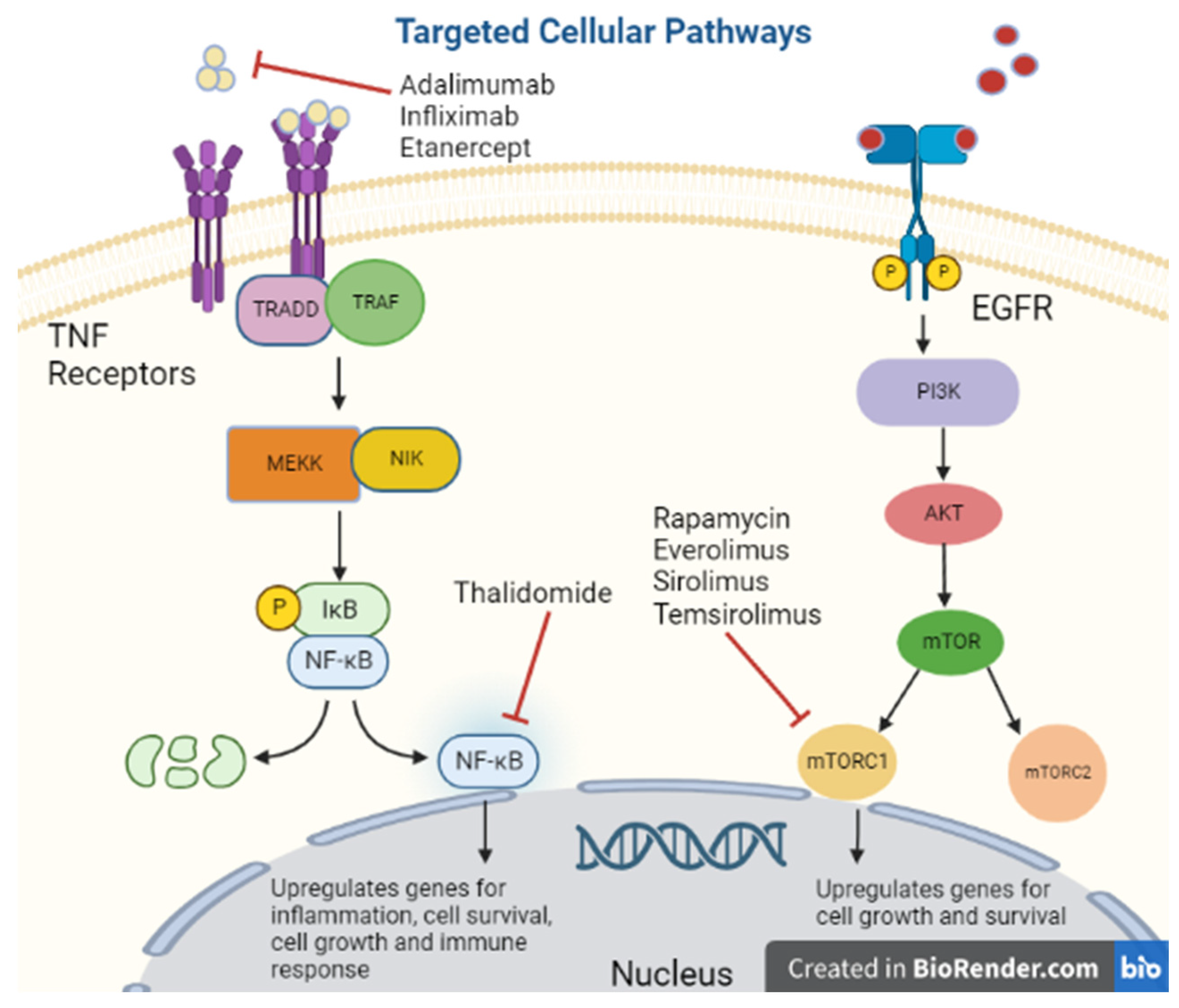
Figure 1. Targeted cellular pathways.
Tumor Necrosis Factor-Alpha Inhibitors
Tumor Necrosis Factor-Alpha [TNF-alpha] has been synonymous with pro-inflammation given its association with pro-inflammatory cytokines IL-1, IL-6, IL-8 and VEGF; therefore, its inhibitors are used for immunosuppression (Figure 2) [29]. However, there is some concern regarding the diverse functionality of TNF-alpha as low levels promoted angiogenesis while higher levels were anti-angiogenetic [30]. Many molecular pathways of TNF-alpha are associated with the upregulation of matrix metallopeptidase 9 [MMP9] which directly degrades the extracellular matrix allowing tumor migration and indirectly promotes cytokines that support cell tumor growth [31]. Despite this mixed data on TNF-alpha, researchers believed blocking its action could be a potential target for malignancy.
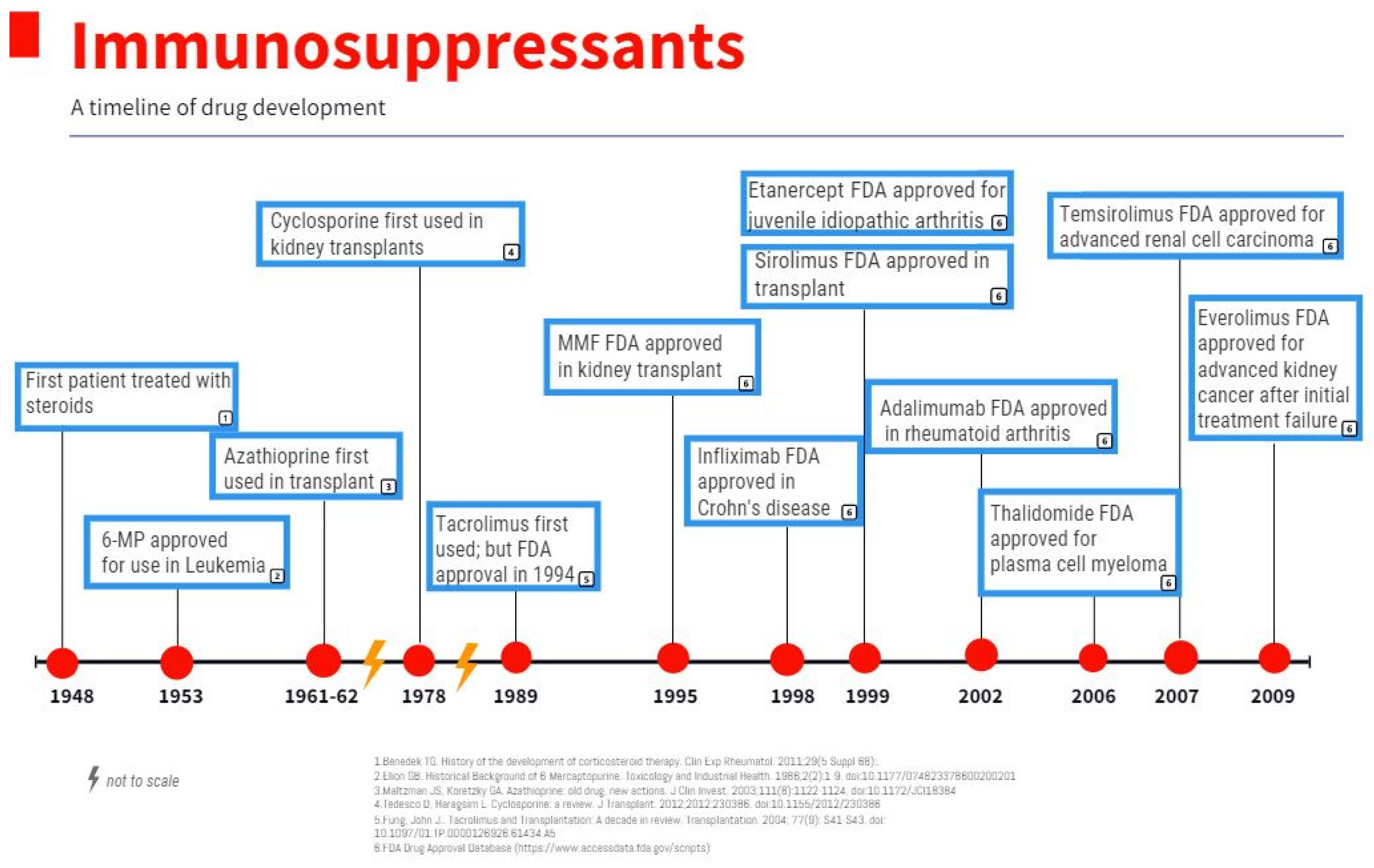
Figure 2. Immunosuppressants: a timeline of drug development.
Thalidomide, an inhibitor of TNF-alpha protein synthesis (Figure 1), has been proven effective against certain cancer types including multiple myeloma, renal, breast, colon, and prostate among others, due to its inhibition of various growth factors, including VEGF, basic FGF, and Hepatocyte Growth Factor, as well as inhibiting tumor DNA synthesis [32][33]. In a clinical trial of 84 patients with refractory myeloma, 76 refractories to high-dose chemotherapy, were treated with escalating doses of Thalidomide to 800 mg over two months, the amount of myeloma protein determined response in serum or Bence Jones protein in urine [34]. Overall, there was a 32% response rate with greater than 90% reduction in 10 of the patients and the majority responded in two months. At 12 month follow up, overall survival was 58%, and event-free survival was 22% [34]. While the data on thalidomide is substantial, other medications in its class had significant adverse effects prompting further investigation. As a class, TNF-alpha inhibitors can promote lymphoproliferative disorders by an unclear mechanism [35]. One study by Askling et al. found an increased risk of leukemia and lymphoma in rheumatoid arthritis patients who were treated with TNF-alpha inhibitors [36]. Another found an increased risk of lymphoma in rheumatoid arthritis patients treated with etanercept or infliximab [37]. There is a dose-dependent relationship between TNF-alpha inhibitor use and the risk of malignancy [38]. Additionally, in a meta-analysis of 36 global clinical trials with 19,041 patients treated with adalimumab [Humira] for rheumatoid arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, psoriatic arthritis, psoriasis, or Crohn’s disease had increased standardized incident ratio of non-melanoma skin cancer and lymphoma collectively [39]. With a few notable exceptions, caution should be used when treating patients with medications that modulate TNF-alpha.
Given this concern for malignancy risk, additional agents were developed with new targets of the inflammatory cascade; namely a monoclonal antibody targeting IL-12 and IL23 [40] and a4b7 integrin antagonist [41]. Contrastingly, both drugs [Ustekinumab and Vedolizumab] were not found to have an increased risk of new or recurrent malignancy in patients with IBD and history of previous malignancy [42][43].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11010099
References
- Burnet, F.M. The Concept of Immunological Surveillance. Prog. Tumor Res. 1970, 13, 1–27.
- Thomas, L.; Lawrence, H. Cellular and Humoral Aspects of the Hypersensitive States; Hoeber-Harper: New York, NY, USA, 1959; pp. 529–532.
- Thomas, L. On immunosurveillance in human cancer. Yale J. Biol. Med. 1982, 55, 329.
- Nozawa, M.; Weil, R.; Mcintosh, R.; Reemtsma, K. Is immunological surveillance not a cell-mediated immune function? Transplantation 1974, 17, 135–139.
- Stutman, O. Chemical carcinogenesis in nude mice: Comparison between nude mice from homozygous matings and heterozygous matings and effect of age and carcinogen dose. J. Natl. Cancer Inst. 1979, 62, 353–358.
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148.
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111.
- Li, C.; Chen, Z. Autoimmunity as an Etiological Factor of Cancer: The Transformative Potential of Chronic Type 2 Inflammation. Front. Cell Dev. Biol. 2021, 9, 664305.
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545.
- Anderson, W.F.; Rabkin, C.S.; Turner, N.; Fraumeni, J.F., Jr.; Rosenberg, P.S.; Camargo, M.C. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J. Natl. Cancer Inst. 2018, 110, 608–615.
- Zityogel, L.; Perreault, C.; Finn, O.J. Beneficial autoimmunity improves cancer prognosis. Nat. Rev. Clin. Oncol. 2021, 18, 591–602.
- Khan, S.; Pruitt, S.; Xuan, L. Prevalence of autoimmune disease among patients with lung cancer: Implications for immunotherapy treatment options. JAMA Oncol. 2016, 2, 1507–1508.
- Jacob, S.; Rahbari, K.; Tegtmeyer, K. Lung Cancer Survival in Patients with Autoimmune Disease. JAMA Netw. Open 2020, 3, e2029917.
- Douglas, C.; Ellershaw, J. Anti-Hu antibodies may indicate a positive response to chemotherapy in paraneoplastic syndrome secondary to small cell lung cancer. Palliat. Med. 2003, 17, 638–639.
- Manley, G.T.; Smitt, P.S.; Dalmau, J.; Posner, J.B. Hu antigens: Reactivity with hu antibodies, tumor expression, and major immunogenic sites. Ann. Neurol. 1995, 38, 102–110.
- Marafini, I.; Monteleone, G.; Stolfi, C. Association Between Celiac Disease and Cancer. Int. J. Mol. Sci. 2020, 21, 4155.
- Singer, P.A.; Cooper, D.S.; Levy, E.G.; Ladenson, P.W.; Braverman, L.E.; Daniels, G.; Greenspan, F.S.; McDougall, I.R.; Nikolai, T.F. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. JAMA 1995, 273, 808–812.
- Rotondi, M.; Groppelli, G.; Croce, L.; Latrofa, F.; Ancona, G.; Coperchini, F.; Pasquali, D.; Cappelli, C.; Fugazza, A.; Guazzoni, V.; et al. Patients with chronic autoimmune thyroiditis are not at higher risk for developing clinically overt thyroid cancer: A 10-year follow-up study. Eur. J. Endocrinol. 2020, 183, 317–323.
- Chen, Y.K.; Lin, C.L.; Cheng, F.T.F.; Sung, F.C.; Kao, C.H. Cancer risk in patients with Hashimoto’s thyroiditis: A nationwide cohort study. Br. J. Cancer 2013, 109, 2496–2501.
- Manger, B.; Schett, G. Paraneoplastic syndromes in rheumatology. Nat. Rev. Rheumatol. 2014, 10, 662–670.
- Soomro, Z.; Youssef, M.; Yust-Katz, S.; Jalali, A. Paraneoplastic Syndromes in Small Cell Lung Cancer. J. Thorac. Dis. 2020, 12, 6253. Available online: https://jtd.amegroups.com/article/view/38423 (accessed on 11 October 2022).
- Mayor, P.C.; Eng, K.H.; Singel, K.L.; Abrams, S.I.; Odunsi, K.; Moysich, K.B.; Fuleihan, R.; Garabedian, E.; Lugar, P.; Ochs, H.D.; et al. Cancer in primary immunodeficiency diseases: Cancer incidence in the United States Immune Deficiency Network Registry. J. Allergy Clin. Immunol. 2018, 141, 1028–1035.
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570.
- Grulich, A.E.; Van Leeuwen, M.T.; Falster, M.O.; Vajdic, C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 2007, 370, 59–67.
- Chandrashekara, S. The treatment strategies of autoimmune disease may need a different approach from conventional protocol: A review. Indian J. Pharm. 2012, 44, 665–671.
- Davidson, A.; Diamond, B. Autoimmune Diseases. N. Engl. J. Med. 2001, 345, 340–350.
- Marmont, A.M. New horizons in the treatment of autoimmune diseases: Immunoablation and stem cell transplantation. Annu. Rev. Med. 2000, 51, 115–134.
- Wallach, D.; Varfolomeev, E.E.; Malinin, N.L.; Goltsev, Y.V.; Kovalenko, A.V.; Boldin, M.P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 1999, 17, 331–367.
- Mocellin, S.; Riccardo Rossi, C.; Pilati, P.; Nitti, D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2004, 16, 35–53.
- Fajardo, L.; Kwan, H.; Kowalski, J.; Prionas, S. Dual role of tumor necrosis factor-alpha in angiogenesis. Am. J. Pathol. 1992, 140, 539–544.
- Overall, C.; Kleifeld, O. Tumour microenvironment-opinion: Validating matrix metalloproteinases as drug targets and antitargets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239.
- Kumar, S.; Witzig, T.E.; Rajkumar, S.V. Thalidomide as an anticancer agent. J. Cell. Mol. Med. 2022, 6, 160–174.
- Teo, S.K. Properties of Thalidomide and its analogues: Implications for anticancer therapy. AAPS J. 2005, 7, E14–E15.
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor Activity of Thalidomide in Refractory Multiple Myeloma. N. Engl. J. Med. 1999, 341, 1565–1571.
- Keystone, E. Advances in targeted therapy: Safety of biological agents. Ann. Rheum. Dis. 2003, 62 (Suppl. 2), ii34–ii36.
- Askling, J.; Fored, C.M.; Baecklund, E.; Brandt, L.; Backlin, C.; Ekbom, A.; Sundström, C.; Bertilsson, L.; Cöster, L.; Geborek, P.; et al. Haematopoietic malignancies in rheumatoid arthritis: Lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann. Rheum. Dis. 2005, 64, 1414.
- Geborek, P.; Bladström, A.; Turesson, C.; Gulfe, A.; Petersson, I.F.; Saxne, T.; Olsson, H.; Jacobsson, L.T. Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann. Rheum. Dis. 2005, 64, 699.
- Solomon, D. The comparative safety and effectiveness of TNFalpha antagonists. J. Manag. Care Pharm. 2007, 13 (Suppl. 1), S7–S18.
- Burmester, G.R.; Mease, P.; Dijkmans, B.A.; Gordon, K.; Lovell, D.; Panaccione, R.; Perez, J.; Pangan, A.L. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann. Rheum. Dis. 2009, 68, 1863–1869.
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and mechanism of ustekinumab: A human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. mAbs 2011, 3, 535–545.
- Wyant, T.; Fedyk, E.; Abhyankar, B. An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab. J. Crohn’s Colitis 2016, 10, 1437–1444.
- Hasan, B.; Tandon, K.S.; Miret, R.; Khan, S.; Riaz, A.; Gonzalez, A.; Rahman, A.U.; Charles, R.; Narula, N.; Castro, F.J. Ustekinumab does not increase risk of new or recurrent cancer in inflammatory bowel disease patients with prior malignancy. J. Gastroenterol. Hepatol. 2022, 37, 1016–1021.
- Hong, S.J.; Zenger, C.; Pecoriello, J.; Pang, A.; Vallely, M.; Hudesman, D.P.; Chang, S.; E Axelrad, J. Ustekinumab and Vedolizumab Are Not Associated With Subsequent Cancer in IBD Patients with Prior Malignancy. Inflamm. Bowel Dis. 2022, 28, 1826–1832.
This entry is offline, you can click here to edit this entry!
