The complex structure of the blood–brain barrier (BBB), which blocks nearly all large biomolecules, hinders drug delivery to the brain and drug assessment, thus decelerating drug development. Conventional in vitro models of BBB cannot mimic some crucial features of BBB in vivo including a shear stress environment and the interaction between different types of cells. There is a great demand for a new in vitro platform of BBB that can be used for drug delivery studies.
1. Structure of BBB and Substance Transportation
1.1. Cellular Structure of BBB
The BBB is a selective physiological barrier that controls transport between the blood and the CNS to maintain homeostasis and the neural microenvironment for optimal brain function [
24]. The BBB is composed of brain microvascular endothelial cells (BMVECs) that are surrounded by pericytes and astrocytes (
Figure 1A). Astrocytes are involved in many biological processes such as the uptake and release of neurotransmitters and the response to stress [
25,
26]. For example, in the case of trauma or pathological tissue injuries, astrocytes are activated and express more glial fibrillary acidic protein and vimentin. Pericytes also play an important role by regulating blood flow [
27]. Moreover, in the BBB, blood vessels are surrounded by the layer of BMVECs and its basement membrane (BM), bordered by epithelial meningeal cells and associated extracellular matrix (ECM) and outer astroglial basement membrane and astrocyte endfeet [
28]. These vascular membranes contribute to the integrity of the blood–brain barrier, forming a three-dimensional protein network mainly composed of laminin, collagen IV isomer, nidogen, and heparan sulfate proteoglycan. These proteins mutually support interactions between brain capillary endothelial cells, pericytes, and astrocytes [
29].
The BOC models have been developed to better simulate real BBB functions. Endothelial cells are a crucial part of the BBB simulation. Different types of endothelial cells have been tested for the construction of BOC. For example, human cardiac microvascular endothelial cells (hCMEC) are favored by many researchers because they are easy to obtain and culture. However, they face the problems of poor barrier leakproofness and low TEER value [
30,
31,
32]. Primary human brain microvascular endothelial cells (hBMEC) isolated from fresh brains are also used, which exhibit a larger endothelial electrical resistance and a higher level of protein expression of the tight junction (TJ), resulting in a more compact structure of the BBB. However, the disadvantages are expensive and less available [
33,
34,
35]. Human umbilical vein endothelial cells (HUVEC) isolated from the umbilical cord, despite their simplicity and accessibility, obtain progressively lower TEER values and loss of function with increasing passages [
34,
36,
37]. Human induced pluripotent stem cell-brain microvascular endothelial cells (hiPSC-BMEC) are derived by inducing stem cell differentiation. Although they demonstrate high TEER values and good formation of TJs, their differentiation process is complex and the allowed passage number is low [
38,
39,
40].
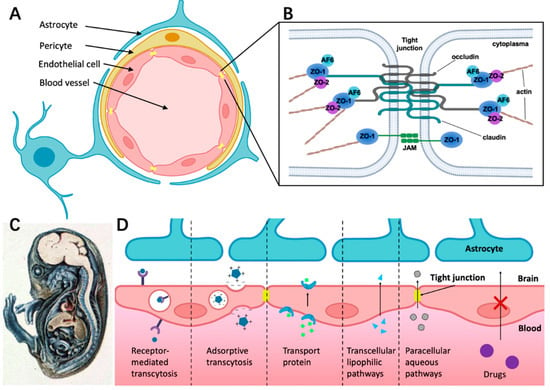
Figure 1. The structure of BBB and different transport pathways for substances across the BBB. (
A) The BBB consists of endothelial cells (ECs) surrounded by pericyte and astrocyte endfeet. (
B) The tight junction is composed of complex and diverse proteins including transmembrane proteins (claudins and occludins), zonula occludens proteins (ZO-I, ZO-II, and ZO-III), and junction-associated molecules (JAMs). (
C) Demonstration of BBB hindering trypan blue entering the CNS in guinea pig embryo. The dye infected almost all of the tissue in the body except most of the brain, which indicates that the CNS is a closed compartment separated from the rest of the embryo [
41]. (
D) Schematic overview of specific transport pathways through the BBB; larger solutes can pass the BBB by receptor-mediated transcytosis and adsorptive transcytosis. Transport protein could cross the BBB through an active transport mechanism. The transcellular lipophilic pathway allows for lipid-soluble agents to pass the BBB. The paracellular pathway enhances the diffusion of water-soluble agents through tight junctions.
1.2. Structure Foundation of Tight Junctions (TJs)
Endothelial cells in the BBB have the main features of extremely low rate of transcytosis and forming restrictive paracellular diffusion barriers, namely tight junctions (TJs), which greatly limits the efficiency of substance transport [
24,
42].
TJ consists of transmembrane proteins including occludin, claudin, the cytoplasmic scaffold proteins ZO-1, -2, and -3, the actin cytoskeleton, and associated signaling proteins (
Figure 1B) [
43]. As shown in
Figure 1B, ZO-1, -2, and -3 link junctional transmembrane proteins such as occludin and claudin to the actin cytoskeleton.
1.3. Different Ways for Substances Crossing the BBB under Physiological Conditions
It is well-known that the BBB has selective permeability. By injecting trypan blue systemically into guinea pig embryos, Wislocki et al. [
41] observed that the dye stained nearly all of the body tissue, except for most of the brain and the spinal cord (
Figure 1C). However, this was not the case when the dye was injected directly into the brain. A diverse combination of physical and chemical barriers constitutes the BBB, which hinders the flow of blood solutes into and out of the brain including drug delivery. Drug transportation across the BBB is complicated by factors such as molecular size, hydrophilicity, intercellular adhesiveness, and efflux transporters (e.g., P-gp) [
44]. As a result of the poor drug transport into the brain, several clinical trials on CNS drugs for Alzheimer’s and Parkinson disease are hindered.
Figure 1D presents different ways for substances crossing the BBB under physiological conditions. Receptor-mediated transcytosis can greatly enhance the efficiency of drug delivery through ligand binding to the membrane receptor of endothelial cells [
44]. Adsorption-mediated transcytosis (AMT) provides another means of delivering drugs to the brain across the BBB. This method offers the potential to bind and uptake cationic molecules to the luminal surface of endothelial cells, followed by exocytosis at the abluminal surface [
45]. Transporter protein such as glucose transporter isoform 1(GLUT-1) could cross the BBB through an active transport mechanism. For example, glucose first binds with the transporter proteins at the blood vessel side of the BBB, then the conformational change of transporter proteins helps transfer glucose or amino acids into the brain side [
46,
47]. The transcellular lipophilic pathway provides approximately 100 cm
2/2 g of brain surface area per average human brain mass for drug delivery [
48]. Furthermore, there is a rare means across the BBB called the paracellular aqueous pathway through which small water-soluble molecules diffuse into the brain [
49].
2. Ultrasound-Driven Microbubbles Reversibly Open BBB
A sound wave with a frequency higher than 20 kHz is called ultrasound, which is widely used in numerous applications, particularly as an imaging tool for clinical diagnosis [
96]. Microbubbles are frequently utilized as ultrasound contrast agents (UCA). These bubbles can be generated by mixing water and gas. They range in size from 1–10 μm, and are encapsulated by a lipid or a protein shell [
97]. Apart from their utilization as UCA, microbubbles also serve as therapeutic agents [
98]. With the advancements in research, ultrasound-driven microbubbles have been found to generate bioeffects that can improve the permeability of the BBB and promote drug uptake [
96].
Due to the high efficiency and low toxicity for local drug delivery, ultrasound-driven microbubbles have demonstrated good potential for delivering drugs to the brain [
99]. Specifically, low acoustic pressure enhances drug uptake by primarily stimulating endocytosis, while high acoustic pressure results in drug uptake by inducing membrane pores [
100,
101].
3. BBB on chip (BOC)
To overcome the major drawbacks of the in vivo model, such as high consumption, ethical issues and large biological differences. There is an urgent need for a preclinical drug screening platform. In recent decades, great efforts have been made to recreate the in vivo environment in vitro. For example, the Transwell model is considered to be a simple way to recreate the BBB in vitro, allowing co-culture of different cell types within the BBB [1]. However, the Transwell model is too simple and static. It lacks mechanical stimuli that are critical for BBB integration [2]. Therefore, a new method was presented, the BOC, a new fabrication led by microfabrication and microfluidics, is an attractive way to reconstruct the BBB in vitro due to its controllability, transparency and ease of resistance measurement and permeability assessment. In addition, it can provide a repeatable and stable environment for drug screening, which could accelerate drug development [3]. Notably, a valid in vitro model must replicate the vital features of the BBB, including physiological and biological properties, and the BOC is considered a potential solution [4].
This entry is adapted from the peer-reviewed paper 10.3390/mi14010112