The epididymis is a convoluted, crescent-shaped structure that connects the testis to the vas deferens and has four main anatomical regions each with unique characteristics and functions: the initial segment, caput (head), corpus (body) and cauda (tail).
- epididymis
- reproduction
- spermatozoa
- sperm transport
- sperm maturation
- sperm motility
1. Introduction
The epididymis is one of the male sex accessory ducts that also include: the seminiferous tubules, rete testis, efferent ducts, vas deferens, ejaculatory duct, and urethra. The epididymis is a convoluted, crescent-shaped structure, about 3.8 cm long in humans, and is conserved in all male reptiles, birds, and mammals [1]. This organ consists of a long tubule that connects the testis to the vas deferens and has four main anatomical regions each with unique characteristics and functions: the initial segment, caput (head), corpus (body) and cauda (tail) (Figure 1) [2].
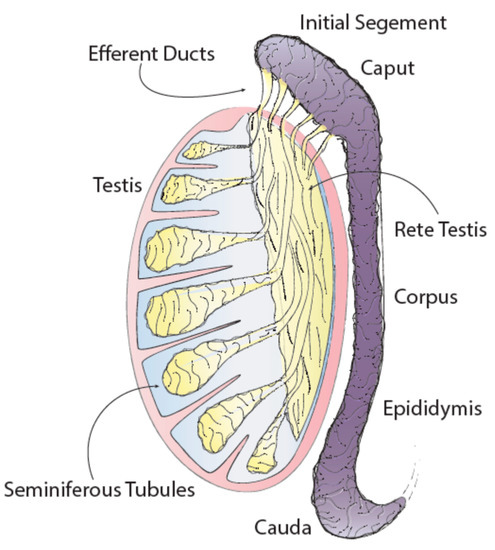
Figure 1. Testis and epididymis anatomy.
2. Structure
To appreciate all of the functions of the epididymis, it is important to understand the structure of the tubules that make up this organ. There are six main cell types that make up the epididymal epithelium, some of these cells are found within all regions of the epididymis while others are localized to specific regions [2]. In general, epididymal cells have high metabolic, endocytic and secretory activity that is primarily regulated by androgens. Androgens are also responsible for regulating the synthesis of some, but not all, proteins that are synthesized and secreted by epididymal cells [21]. Principal cells are the major cell type in the epididymal epithelium and exist along the entire epididymal duct. Depending on the region, principal cells account for between 65 and 80% of the epididymal epithelium. These cells are primarily responsible for absorption and secretion of materials into the epididymal lumen and therefore have high secretory and endocytic activity [2][22]. Additionally, principal cells are the site of production and release of cargo-containing epididymosomes [5]. Apical cells are primarily located at the initial segment of the epididymal epithelium and also have endocytic activity. Narrow cells also exist exclusively within the initial segment and are, as the name indicates, narrower than adjacent principal cells. These cells have been shown to secrete H+ ions into the epididymal lumen, and are responsible for endocytosis [22]. Clear cells are another cell type with high endocytic activity; however, these cells are found exclusively within the caput, corpus and cauda regions of the epididymis and are not located within the initial segment. Clear cells are the primary cell type responsible for taking up cytoplasmic droplets that are released from sperm cells during maturation in the epididymal lumen. Together, clear and narrow cells are thought to be the primary cells responsible for the regulation of luminal pH [22]. Basal cells are located along the tubule and adhere to the basement membrane [2]. These cells are an integral part of tubule structure and it has been suggested that they may indirectly affect luminal environment by regulating some principal cell functions [2][22][23]. Finally, halo cells exist throughout the epididymal epithelium and are the primary immune cells in the epididymis [2]. The epididymal epithelium is also surrounded by smooth muscle which is thinnest at the caput and gets progressively thicker towards the cauda epididymis. In fact, the cauda is surrounded by two unique smooth muscle layers while the caput is encapsulated by a single layer [24].
3. Functions
3.1. Sperm Transport
The most obvious function of the epididymis is to transport sperm from the rete testes to the vas deferens. Total transit time through the epididymis is generally between 10–15 days [2]. Transport is achieved primarily by rhythmic contractions of the smooth muscle layers surrounding the epididymis. While contractions occur most frequently at the caput, they are most amplified at the cauda. Additionally, it has been suggested that cilia on epididymal epithelial cells may aid in directing sperm transit through the epididymis [24].
3.2. Sperm Concentration
The main process that occurs in the initial segment of the epididymis is the absorption of fluid by epithelial cells. The efferent ducts and initial segment are responsible for absorbing approximately 90% of the fluid that leaves the rete testis [2]. Additional absorption occurs throughout the remainder of epididymal transit, resulting in a dramatic increase in sperm concentration in the cauda epididymis as compared to the rete testis [25]. Sperm concentration in the epididymis is necessary for increased sperm concentration in semen, an important factor in male fertility.
3.3. Sperm Protection
An additional function of the epididymis is to protect sperm cells during epididymal transit from damage caused by the external environment [26]. The epididymis has many mechanisms to aid in the protection of sperm. Epididymal epithelial cells have high metabolic activity which results in the generation of reactive oxygen species that are harmful to sperm cells. To combat this problem, epithelial cells excrete various antioxidant enzymes, including superoxide dismutase, into the epididymal lumen to neutralize reactive oxygen species [27]. Additionally, a blood-epididymis barrier functions to shield maturing sperm cells from the immune system and from harmful substances that might exist in the bloodstream [2].
3.4. Sperm Storage
The cauda epididymis functions as a storage location for functionally mature sperm cells prior to ejaculation [22]. At a given time, between 50 and 80% of sperm in the epididymal lumen are located in the cauda epididymis, depending on species [2]. Epithelial cells of the cauda secrete factors that help to maintain a luminal environment designed to maintain sperm in a quiescent state during storage. While many factors relating to this quiescent state are still unknown, regulation of luminal pH and the presence of specific proteins and enzymes are thought to play a role [2]. After ejaculation, sperm leave this quiescent state, and metabolic activity increases 3–5 fold as compared to activity in the cauda epididymis [28].
3.5. Sperm Maturation
An essential process required for normal male fertility that occurs during epididymal transit is sperm maturation. Sperm undergo many maturational changes during this time, but most importantly they acquire motility and factors necessary for successful fertilization of an oocyte. The process of maturation occurs via direct contact of sperm with the contents of the epididymal lumen environment. Luminal environment is specific to each region of the epididymis and differences between regions are due to the varied cell composition of the epithelium and hormonal regulation, among other factors [22]. As sperm progress through the epididymis, they undergo changes in nuclear compaction, plasma membrane composition, cytoskeletal structure, protein payload and non-coding RNA payload [22][29].
3.6. Acquisition of Motility
Testicular sperm are considered immotile. While these cells may twitch, they are unable to complete any progressive movement. By the time spermatozoa reach the cauda epididymis a majority of cells are capable of progressive motility. Motility is thought to be intrinsic to sperm cells and can be artificially developed in certain conditions [20]. The epididymal lumen, however, provides the best environment for this activation to take place. From a morphological and structural perspective, many changes to sperm occur which help to facilitate motility [2]. Sperm plasma membrane composition is altered throughout epididymal transit resulting in a narrowing of the sperm acrosome. Alterations in membrane composition are thought to be driven by concentration gradients of specific enzymes and molecules along the tubule lumen [20][22]. Increased numbers of disulfide bridges in the sperm nucleus result in compaction of genetic material and the sperm head. Additionally, the cytoplasmic droplet, which is eventually shed upon ejaculation, migrates caudally along sperm during epididymal transit, and this droplet has been implicated in affecting some biochemical aspects of motility. Importantly, some signaling pathways have been associated with sperm motility, and evidence suggests that sperm may have functional flagellar machinery that is activated during epididymal transit [20].
3.7. Fertilization Capabilities
In addition to the development of motility, sperm also gain factors necessary for fertilization of an oocyte during maturation in the epididymis. Specifically, they acquire factors necessary for binding and penetrating the cumulus cells and zona pellucida. It has been well established that the primary mechanism responsible for sperm-oocyte binding is carbohydrate-protein interactions between oligosaccharides on the oocyte membrane and receptor proteins on the sperm membrane [30]. The sperm acrosome reaction is required for sperm penetration and takes place within the fallopian tube. Many factors work in concert to initiate and complete the acrosome reaction, and oocyte-sperm carbohydrate-protein interactions are a key factor in this process [18]. Multiple proteins on the sperm surface are important for sperm-zona binding, including ZP proteins, acrosin binding protein and CRISP1, among others [18][19][31][32]. Binder of SPerm (BSP) family proteins also play a role in this process by stabilizing the sperm membrane and regulating timing of sperm capacitation [33]. Multiple ADAM family proteins are also important in sperm-zona binding, and while many of these proteins are expressed in the testis, some studies have shown increased levels of these proteins in sperm after epididymal transit [34][35][36]. Studies assessing the sperm proteome throughout epididymal transit have shown that these proteins, among others, are acquired by maturing sperm during epididymal transit [20][22][32][34][35][37][38]. These data, as well as early works assessing the fertilization capabilities of caput sperm for in vitro fertilization provide evidence for the epididymis having an essential role in sperm competency.
4. Sperm Maturation and Reproduction
New and historical data point to the importance of proper sperm maturation in successful reproduction. In fact, evidence suggests that the consequence of immature sperm on reproductive potential may go beyond a reduced ability to successfully fertilize an oocyte and extend into the support of early embryogenesis.
Historically, gross defects in embryogenesis from caput-sperm-fertilized oocytes have been well documented [2][39][40][41][42]. In the past, in vitro fertilization (IVF) was used in animal models as a method to study the effects of using immature (caput) sperm for reproduction. Due to these cells not having undergone complete epididymal transit, they lacked many of the necessary fertilization factors, and therefore fertilization rates in these studies were very low. In those embryos where fertilization did occur, however, gross defects in embryonic development were observed. Early studies in multiple model systems have suggested that sperm acquire fertilization factors relatively early during epididymal transit, but that the ability to produce viable offspring and full litters is not gained until later in the epididymis [2][39][40][41][42]. In 1977, a study was conducted where rabbit oocytes were fertilized with either immature corpus sperm or cauda sperm. These authors found that in corpus-derived embryos the first cleavage was consistently delayed as compared to cauda-derived embryos [41]. Likewise, in 1990, caput and cauda sperm were compared in an IVF study using mice. In successfully fertilized caput embryos, only approximately 8% developed to the blastocyst stage compared to approximately 48% of cauda-derived embryos [42].
Developments in assisted reproductive technologies have since allowed additional exploration into these observations, specifically with the use of intracytoplasmic sperm injection (ICSI) which does not require sperm to be motile, nor possess fertilization factors. A recent study by Conine et al. displayed striking results where ICSI embryos fertilized with caput sperm displayed significantly reduced implantation rates and ultimately 100% embryonic lethality [43]. These results were attributed to non-coding, small RNA (sRNA) payloads in caput sperm, as these payloads undergo significant remodeling during epididymal transit [43][44][45]. However, some data from other groups are in disagreement with these results, where authors successfully generated ICSI offspring using caput sperm [46][47]. Even considering inconsistencies in results, both historical and recent data suggest sperm competency to support normal embryogenesis may be acquired during epididymal transit. Recently, it has been suggested that cargo contained in epididymosomes and delivered to sperm during epididymal transit may be responsible for the acquisition of these critical factors [3][43][45].
This entry is adapted from the peer-reviewed paper 10.3390/ijms21155377
References
- Jones, R.; Lopez, K.H. Human Reproductive Biology, 4th ed.; Academic Press: Cambridge, NY, USA, 2004.
- Robaire, B.; Hinton, B.T.; Orgebin-Crist, M. CHAPTER 22—The Epididymis. In Knobil and Neill’s Physiology of Reproduction, 3rd ed.; Neill, K., Ed.; Academic Press: Cambridge, NY, USA, 2006.
- Nixon, B.; De Iuliis, G.N.; Dun, M.D.; Zhou, W.; Trigg, N.A.; Eamens, A.L. Profiling of epididymal small non-protein-coding RNAs. Andrology 2019, 7, 669–680.
- Sullivan, R. Epididymosomes: A heterogeneous population of microvesicles with multiple functions in sperm maturation and storage. Asian J. Androl. 2015, 17, 726–729.
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35.
- Trigg, N.A.; Eamens, A.L.; Nixon, B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction 2019, 157, R209–R223.
- Abbot, P.; Capra, J.A. What is a placental mammal anyway? Elife 2017, 6, e30994.
- Wildman, D.E.; Chen, C.; Erez, O.; Grossman, L.I.; Goodman, M.; Romero, R. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc. Natl. Acad. Sci. USA 2006, 103, 3203–3208.
- Saitou, M.; Miyauchi, H. Gametogenesis from Pluripotent Stem Cells. Cell Stem Cell 2016, 18, 721–735.
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17.
- Jauregui, E.J.; Mitchell, D.; Topping, T.; Hogarth, C.A.; Griswold, M.D. Retinoic acid receptor signaling is necessary in steroidogenic cells for normal spermatogenesis and epididymal function. Development 2018, 145.
- Gilbert, S.F. Oogenesis. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000.
- Virant-Klun, I. Postnatal oogenesis in humans: A review of recent findings. Stem Cells Cloning 2015, 8, 49–60.
- McGee, E.A.; Raj, R.S. Regulators of ovarian preantral follicle development. Semin. Reprod. Med. 2015, 33, 179–184.
- Zhai, F.M.X.; Yan, L.; Qiao, J. The Molecular Genetics of Oogenesis. In Human Reproductive and Prenatal Genetics; Elsevier Inc: New York, NY, USA, 2019.
- Robker, R.L.; Hennebold, J.D.; Russell, D.L. Coordination of Ovulation and Oocyte Maturation: A Good Egg at the Right Time. Endocrinology 2018, 159, 3209–3218.
- Yeung, C.-H.; Cooper, T.G. Acquisition and Development of Sperm Motility Upon Maturation in the Epididymis. In The Epididymis: From Molecules to Clinical Practice: A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens; Robaire, B., Hinton, B.T., Eds.; Springer US: Boston, MA, USA, 2002; pp. 417–434.
- Yeste, M.; Jones, C.; Amdani, S.N.; Coward, K. Oocyte Activation and Fertilisation: Crucial Contributors from the Sperm and Oocyte. Results Probl. Cell Differ. 2017, 59, 213–239.
- Topfer-Petersen, E.; Petrounkina, A.M.; Ekhlasi-Hundrieser, M. Oocyte-sperm interactions. Anim. Reprod. Sci. 2000, 60–61, 653–662.
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology 2017, 5, 204–218.
- Brooks, D.E. Epididymal functions and their hormonal regulation. Aust. J. Biol. Sci. 1983, 36, 205–221.
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2009, 15, 213–227.
- Leung, G.P.; Cheung, K.H.; Leung, C.T.; Tsang, M.W.; Wong, P.Y. Regulation of epididymal principal cell functions by basal cells: Role of transient receptor potential (Trp) proteins and cyclooxygenase-1 (COX-1). Mol. Cell Endocrinol. 2004, 216, 5–13.
- Elfgen, V.; Mietens, A.; Mewe, M.; Hau, T.; Middendorff, R. Contractility of the epididymal duct: Function, regulation and potential drug effects. Reproduction 2018, 156, R125–R141.
- Turner, T.T. Resorption versus secretion in the rat epididymis. J. Reprod. Fertil. 1984, 72, 509–514.
- Hinton, B.T.; Palladino, M.A.; Rudolph, D.; Lan, Z.J.; Labus, J.C. The role of the epididymis in the protection of spermatozoa. Curr. Top. Dev. Biol. 1996, 33, 61–102.
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668.
- Jones, R.C. To store or mature spermatozoa? The primary role of the epididymis. Int. J. Androl. 1999, 22, 57–67.
- Sullivan, R.; Mieusset, R. The human epididymis: Its function in sperm maturation. Hum. Reprod. Update 2016, 22, 574–587.
- Maldera, J.A.; Weigel Munoz, M.; Chirinos, M.; Busso, D.; Raffo, F.G.E.; Battistone, M.A.; Blaquier, J.A.; Larrea, F.; Cuasnicu, P.S. Human fertilization: Epididymal hCRISP1 mediates sperm-zona pellucida binding through its interaction with ZP3. Mol. Hum. Reprod. 2014, 20, 341–349.
- Thaler, C.D.; Cardullo, R.A. The initial molecular interaction between mouse sperm and the zona pellucida is a complex binding event. J. Biol. Chem. 1996, 271, 23289–23297.
- Cohen, D.J.; Maldera, J.A.; Vasen, G.; Ernesto, J.I.; Munoz, M.W.; Battistone, M.A.; Cuasnicu, P.S. Epididymal protein CRISP1 plays different roles during the fertilization process. J. Androl. 2011, 32, 672–678.
- Plante, G.; Manjunath, P. Epididymal Binder of SPerm genes and proteins: What do we know a decade later? Andrology 2015, 3, 817–824.
- Oh, J.S.; Han, C.; Cho, C. ADAM7 is associated with epididymosomes and integrated into sperm plasma membrane. Mol. Cells 2009, 28, 441–446.
- Skerget, S.; Rosenow, M.A.; Petritis, K.; Karr, T.L. Sperm Proteome Maturation in the Mouse Epididymis. PLoS ONE 2015, 10, e0140650.
- Wong, G.E.; Zhu, X.; Prater, C.E.; Oh, E.; Evans, J.P. Analysis of fertilin alpha (ADAM1)-mediated sperm-egg cell adhesion during fertilization and identification of an adhesion-mediating sequence in the disintegrin-like domain. J. Biol. Chem. 2001, 276, 24937–24945.
- Caballero, J.N.; Frenette, G.; Belleannee, C.; Sullivan, R. CD9-positive microvesicles mediate the transfer of molecules to Bovine Spermatozoa during epididymal maturation. PLoS ONE 2013, 8, e65364.
- Sullivan, R.; Frenette, G.; Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 2007, 9, 483–491.
- Orgebin-Crist, M.C. Sperm maturation in rabbit epididymis. Nature 1967, 216, 816–818.
- Orgebin-Crist, M.C. Studies on the function of the epididymis. Biol. Reprod. 1969, 1, 155–175.
- Orgebin-Crist, M.C.; Jahad, N. Delayed cleavage of rabbit ova after fertilization by young epididymal spermatozoa. Biol. Reprod. 1977, 16, 358–362.
- Wazzan, W.C.; Gwatkin, R.B.; Thomas, A.J., Jr. Zona drilling enhances fertilization by mouse caput epididymal sperm. Mol. Reprod. Dev. 1990, 27, 332–336.
- Conine, C.C.; Sun, F.; Song, L.; Rivera-Perez, J.A.; Rando, O.J. Small RNAs Gained during Epididymal Transit of Sperm Are Essential for Embryonic Development in Mice. Dev. Cell 2018, 46, 470–480.
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396.
- Sharma, U.; Sun, F.; Conine, C.C.; Reichholf, B.; Kukreja, S.; Herzog, V.A.; Ameres, S.L.; Rando, O.J. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev. Cell 2018, 46, 481–494.
- Suganuma, R.; Yanagimachi, R.; Meistrich, M.L. Decline in fertility of mouse sperm with abnormal chromatin during epididymal passage as revealed by ICSI. Hum. Reprod. 2005, 20, 3101–3108.
- Zhou, D.; Suzuki, T.; Asami, M.; Perry, A.C.F. Caput Epididymidal Mouse Sperm Support Full Development. Dev. Cell 2019, 50, 5–6.
