Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Carbon nanoparticles (CNPs) have demonstrated utility in a wide range of biological applications such as imaging, sensing and as surface-coatings. Biomass waste can be derived from either plant or animal matter as a result of processing higher-value materials, for instance leaves from trees as by product from processed wood or paper production. Biomass is abundant: trees, agriculture crops, energy crops, fruits, vegetation, wood, aquatic plants and algae, general municipal waste and animal waste.
- green synthesis
- carbon nanomaterials
- biomass
1. Introduction
Carbon nanoparticles (CNPs) have demonstrated utility in a wide range of biological applications such as imaging, sensing and surface-coating. They enjoy a growing range of applications in the drug delivery of a number of biomolecules such as DNA, antibodies, and proteins. CNPs are considered the ideal candidates for metal-based sensor applications and rapid diagnostic assays due, in the main part, to their high fluorescence value. As such, they can displace gold, colored latex, silica, quantum dots, or phosphor nanoparticles, in relevant applications (Figure 1) [1][2][3][4][5].
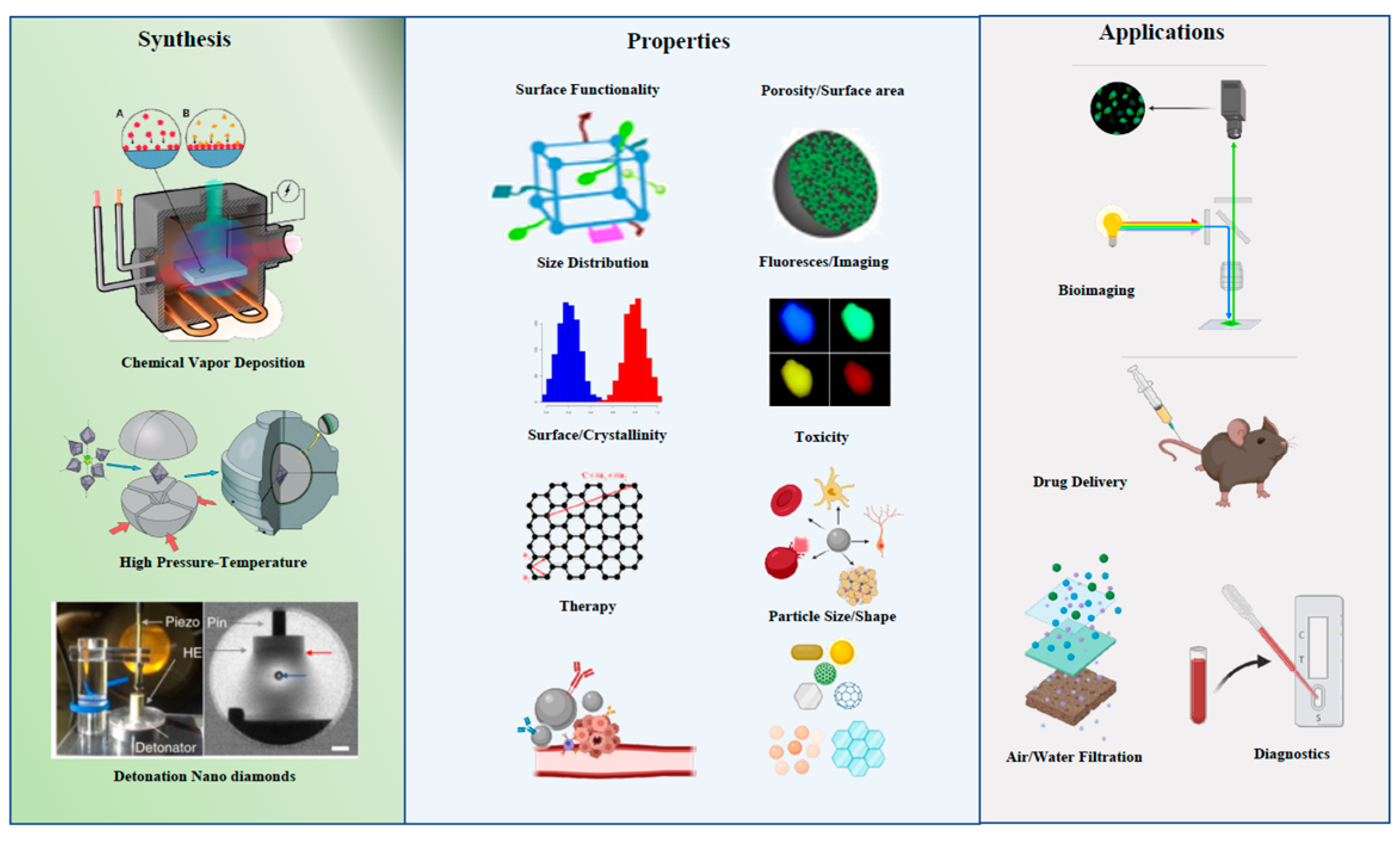
Figure 1. Synthesis, properties and applications of carbon nanoparticles.
CNPs have a range of highly desirable attributes; they exhibit low toxicity, high biocompatibility, can be readily suspended into solution [6] and can be modified post-production with a range of chemical functionality. Carbon based materials already play a critical role in many applications: electro-catalysts, electrodes in storage devices, biofuels, heterogenous catalysis and photo catalysis [7]. Carbonaceous materials form the basis of gas storage (e.g., CO2), hydrogen capture, water purification and as additives to rectify soil properties [2]. The field of optical sensing has been extended with the discovery of highly fluorescent CNPs and carbon nanodots (CNDs) [8][9]. CNP and CND probes demonstrate remarkable properties; emission characteristics that are tunable based on particle size, high emission quantum yields, physical and chemical stability, narrow spectral bands and optimization of the surface to effect selective sensing applications [10]. Other than optical sensing, these CNPs have also seen use in applications such as photocatalysis, bioimaging and optoelectronics [11][12][13]. A typical example of optical sensing is the light-emitting properties of semiconductor quantum dots (QDs) used for in vitro and in vivo bioimaging [14][15][16] (Figure 2). QD’s strong optical absorption peaks are very sensitive to the surrounding environment and medium, which enables biomolecule calorimetry to be completed [17][18]. Another significant advantage of CNPs is that they can be produced economically with high purity and are readily fabricated from naturally occurring biomass and generated utilizing green chemistry [19].
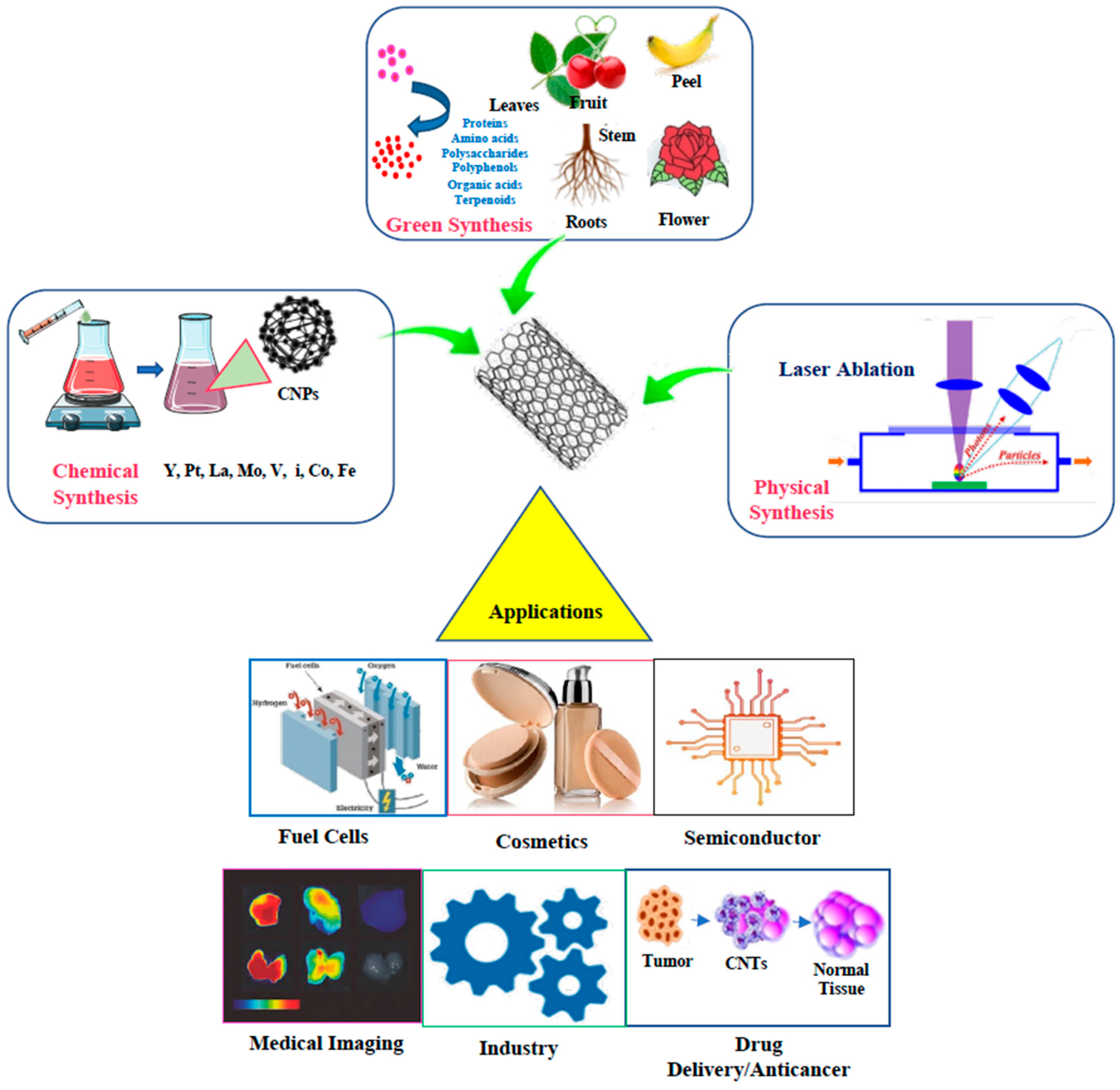
Figure 2. Types of syntheses of CNPs and example applications.
Since the pioneering studies by Sumio Iijima in 1991 with the characterization of carbon nanotubes, CNPs have been used in a number of applications. The impact of CNPs has been recognized internationally with researchers receiving the highest of scientific awards [20]. More recently, due to their adoption in the fields of medical, environmental and novel materials, industry has paved the way for the rapid development of a variety of CNPs, such as carbon nanotubes (CNTs), QDs, graphene and nano diamonds (NDs). Based on the lattice arrangement of the main building unit carbon, CNPs are classified as either one-dimensional (1D), two-dimensional (2D) or three-dimensional (3D) structures. For instance, CNTs are 1D, graphene is 2D and NDs have a 3D structure [21][22]. Among these CNPs, CNTs are the hardest materials due to their extended aromatic C-C bond network. The arrangement of these single C-C network layers may be classified further into either single-wall carbon nanotubes (SWCNTs) or multiwall carbon nanotubes (MWCNTs) [23][24]. This C-C bond network gives a honeycomb-like arrangement. SWCNTs and MWCNTs have inter-layer distances of 0.32–0.35 nm and 2 to 50 sheets are found arranged in the tubules. This gives rise to a wide range of wall thicknesses [25]. In the case of graphene, discovered recently in single-layer form as reported by Novoselov [26], a hexagonal arrangement is generated through an extended sp2 hybridized network.
Production of CNPs may be achieved through chemical vapor deposition (CVD), arc discharge vaporization, floating catalytic methods, laser ablation/evaporation and low temperature solid pyrolysis (Figure 2) [27][28][29][30]. However, accepted methods for the synthesis of CNPs have environmental concerns such as the high consumption of raw materials and the use of strong acids. In addition, CNP containing materials produced using these methods exhibits a limited capacity for the loading of metals oxides or post-generation modification of the particle surface [31][32]. Therefore, the focus of CNP production has moved towards establishing methods that are less demanding of resources, low-cost and eco-friendly [33].
In a green-synthesis approach, biomass such as woods, leaves and low-value biomaterials, such as plant husks, have been used as the precursor for developing carbon materials. In some cases, green synthesis is achieved through using biomass as the starting material, which can also replace the metal catalysts used in CNP fabrication. Based on the reaction conditions and precursor materials, different forms of CNPs such as nanofibers, nanotubes or nanoporous configurations can be generated. For instance, allotropic forms of graphitic carbon nanostructures, with a coil morphology, were fabricated by a precipitation method at 900 °C via the hydrothermal treatment of cellulose [34]. In addition, carbon nanosheets can be fabricated from lignocellulosic biomass derived from coconut coir through the action of hydrothermal carbonization followed by pyrolysis [35]. The effect of clay mineral particles has also been evaluated [36][37]. Similarly, cellulose (husk) has been used to develop carbon hollow nanostructures via a three step acid digestion process, followed by charring and high temperature pyrolysis (CO2 laser 2200 °C) [38]. Many other types of food and agricultural waste such as proteins, chitin, lignin, carbohydrates, hemicellulose, and honeycomb have been used in the fabrication of CNPs using a green synthesis approach [19][39][40].
2. Processing of Biomass
Biomass waste can be derived from either plant or animal matter as a result of processing higher-value materials, for instance leaves from trees as by product from processed wood or paper production [41]. Biomass is abundant: trees, agriculture crops, energy crops, fruits, vegetation, wood, aquatic plants and algae, general municipal waste and animal waste [7][42]. In general, such biomass materials are subjected to various processing methods to obtain energy and carbon allotropes. Two broad classifications may be made: biochemical processing (e.g., anaerobic digestion and fermentation) and thermochemical processing (e.g., pyrolysis, combustion and gasification) [43].
The process of pyrolysis can be a highly efficient energy recovery process and has the potential to produce products ranging from char, to gas and oil [44]. Char as a by-product of energy recovery processes can act as a source for various carbon materials such as activated carbon, porous carbon and CNPs such as CNTs, graphene and fullerenes, all of which can be generated through controlled green synthesis processes [45][46][47][48]. These CNPs can be functionalized further and their surface texture and functionality modified by using different surface treatment agents and activation methods [49][50]. Final products find a wide range of applications such as environmental sensors, water purification, hydrogen capture and storage, energy conversion and air pollution control [50][51]. Generally, to obtain CNPs from the by-product of biomass processing three types of treatments are used: physical activation, chemical activation and hydrothermal carbonization (HTC) [20][52].
Physical activation is a two-step process; raw materials are subjected to pyrolysis and carbonization at a temperature below 1000 K, and in second step, subjected to controlled gasification process at high temperatures above 1150 K, in the presence of oxidizing gases (CO2, air, steam or a mixture of these) [53]. With steam or CO2 as an activation gas, equipment is easy to clean, and the removal of the oxidant is straightforward. Various biomasses such as rice straw, peanut, rice husk, corn hulls, corncob, coconut shells, pecan shell and almond shells have all been used to developed CNPs using this physical activation method [54][55][56][57][58].
With chemical activation, a well-established single step is undertaken where a precursor is mixed with a chemical activation agent (H3PO4, ZnCl2, KOH, K2CO3, etc.) and when heated to temperatures ranging from 700 K to 1200 K, carbonization and activation occur simultaneously [59][60][61][62]. The chemical activation process results in carbon materials with high porosity and surface area (>2000 m2 g−1), and larger pore sizes [41]. The chemical activation process has advantages compared to physical activation as it is faster, requires lower conversion temperatures, is higher in carbon yield and provides a more uniformly high-porosity material. Among chemical agents, KOH is favored and since 1978 active carbon produced from KOH treatment processes has generated material with uniform porosity and a high surface area (up to 3000 m2 g−1) [63].
The process of hydrothermal carbonization (HTC) is inspired by natural processes in which biological materials undergo a long, natural chemical coaling process. The application of high pressure and heat converts biomass to peat or coal over thousands to millions of years in a natural phenomenon [40]. HTC is the direct chemical imitation of this natural process but occurring over a much shorter timeframe. This process was first reported by Bergius in 1913 and remodeled by Berl and Schmidt in 1932, which is the well-known methodology for converting cellulose to activated carbon, and is still in common use today [63]. Recently, this method was shown to produce carbon materials from biomass with much milder conditions using temperatures below 500 K, pure water and self-steam pressure [40][49]. This process is considered both physical and chemical processing and is desirable due to the comparatively low temperature, cost effectiveness and overall eco-friendly synthesis. Carbohydrates and their derivatives such as hydroxymethyl furfural, glucose, xylose, furfural, sucrose and starch have all been converted to carbonaceous materials with HTC using a temperature of only 180 °C [64]. HTC can generate porous materials directly from biomass but, as compared to the chemical process, a less porous and lower surface area product results [40][65]. Therefore, such carbon materials are not optimal for the applications of chemical or gas adsorption, or catalysis and energy storage. To improve the porosity of carbon materials from HTC, different templates (e.g., SPA-15) or additives (and therefore more chemical-process aligned methods) such as KOH are used [66][67].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24021023
References
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323.
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656.
- Sagbas, S.; Sahiner, N. Carbon dots: Preparation, properties, and application. In Nanocarbon and Its Composites; Khan, A., Jawaid, M., Mohamed Asiri, I., Mohamed Asiri, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 651–676.
- Mendes, R.G.; Bachmatiuk, A.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Carbon nanostructures as multi-functional drug delivery platforms. J. Mater. Chem. B 2013, 1, 401–428.
- Liu, Y.; Dong, X.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307.
- Manzetti, S.; Gabriel, J.-C.P. Methods for dispersing carbon nanotubes for nanotechnology applications: Liquid nanocrystals, suspensions, polyelectrolytes, colloids and organization control. Int. Nano Lett. 2019, 9, 31–49.
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717.
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744.
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686.
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253.
- Liu, L.; Li, Y.; Zhan, L.; Liu, Y.; Huang, C. One-step synthesis of fluorescent hydroxyls-coated carbon dots with hydrothermal reaction and its application to optical sensing of metal ions. Sci. China Chem. 2011, 54, 1342–1347.
- Wee, S.S.; Ng, Y.H.; Ng, S.M. Synthesis of fluorescent carbon dots via simple acid hydrolysis of bovine serum albumin and its potential as sensitive sensing probe for lead (II) ions. Talanta 2013, 116, 71–76.
- Wang, Y.X.Y.L.; Ming, Z. Research on seismic behavior and cost estimation of a new concrete filled steel tube frame structure. Adv. Mat. Res. 2011, 335, 1231–1234.
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446.
- Efros, A.L.; Nesbitt, D.J. Origin and control of blinking in quantum dots. Nat. Nanotechnol. 2016, 11, 661–671.
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486.
- Ali, M.R.K.; Wu, Y.; Ghosh, D.; Do, B.H.; Chen, K.; Dawson, M.R.; Fang, N.; Sulchek, T.A.; El-Sayed, M.A. Nuclear Membrane-Targeted Gold Nanoparticles Inhibit Cancer Cell Migration and Invasion. ACS Nano 2017, 11, 3716–3726.
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319.
- Roshni, V.; Ottoor, D. Synthesis of carbon nanoparticles using one step green approach and their application as mercuric ion sensor. J. Lumin. 2015, 161, 117–122.
- Shen, Y. Carbothermal synthesis of metal-functionalized nanostructures for energy and environmental applications. J. Mater. Chem. A 2015, 3, 13114–13188.
- Villarreal, C.C.; Pham, T.; Ramnani, P.; Mulchandani, A. Carbon allotropes as sensors for environmental monitoring. Curr. Opin. Electrochem. 2017, 3, 106–113.
- Bhattacharya, D.; Jana, D. Twin T-graphene: A new semiconducting 2D carbon allotrope. Phys. Chem. Chem. Phys. 2020, 22, 10286–10294.
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon Nanotubes—The Route toward Applications. Science 2002, 297, 787–792.
- Ni, Z.; Li, Q.; Yan, L.; Gong, J.; Zhu, D. Welding of multi-walled carbon nanotubes by ion beam irradiation. Carbon 2008, 46, 376–378.
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669.
- Tan, G.; Mieno, T. Synthesis of single-walled carbon nanotubes by arc-vaporization under high gravity condition. Thin Solid Films 2010, 518, 3541–3545.
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.-Q.; Xu, Z.; Peng, F.; et al. Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 2014, 510, 522–524.
- Liu, J.; Lu, J.; Lin, X.; Tang, Y.; Liu, Y.; Wang, T.; Zhu, H. The electronic properties of chiral carbon nanotubes. Comput. Mater. Sci. 2017, 129, 290–294.
- Ci, L.; Li, Y.; Wei, B.; Liang, J.; Xu, C.; Wu, D. Preparation of carbon nanofibers by the floating catalyst method. Carbon 2000, 38, 1933–1937.
- Ateia, M.; Koch, C.; Jelavić, S.; Hirt, A.; Quinson, J.; Yoshimura, C.; Johnson, M. Green and facile approach for enhancing the inherent magnetic properties of carbon nanotubes for water treatment applications. PLoS ONE 2017, 12, e0180636.
- Chen, B.; Zhu, Z.; Ma, J.; Yang, M.; Hong, J.; Hu, X.; Qiu, Y.; Chen, J. One-pot, solid-phase synthesis of magnetic multiwalled carbon nanotube/iron oxide composites and their application in arsenic removal. J. Colloid Interface Sci. 2014, 434, 9–17.
- Thambiraj, S.; Shankaran, D.R. Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl. Surf. Sci. 2016, 390, 435–443.
- Sevilla, M.; Fuertes, A.B. Graphitic carbon nanostructures from cellulose. Chem. Phys. Lett. 2010, 490, 63–68.
- Omoriyekomwan, J.E.; Tahmasebi, A.; Dou, J.; Wang, R.; Yu, J. A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass. Fuel Process. Technol. 2021, 214, 106686.
- Barin, G.B.; Gimenez, I.D.F.; da Costa, L.P.; Filho, A.G.S.; Barreto, L.S. Hollow carbon nanostructures obtained from hydrothermal carbonization of lignocellulosic biomass. J. Mater. Sci. 2013, 49, 665–672.
- Barin, G.B.; Gimenez, I.D.F.; da Costa, L.P.; Filho, A.G.S.; Barreto, L.S. Influence of hydrothermal carbonization on formation of curved graphite structures obtained from a lignocellulosic precursor. Carbon 2014, 78, 609–612.
- Herring, A.M.; McKinnon, J.T.; McCloskey, B.D.; Filley, J.; Gneshin, K.W.; Pavelka, R.A.; Kleebe, H.-J.; Aldrich, D.J. A Novel Method for the Templated Synthesis of Homogeneous Samples of Hollow Carbon Nanospheres from Cellulose Chars. J. Am. Chem. Soc. 2003, 125, 9916–9917.
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290.
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M.-M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828.
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2017, 5, 69–88.
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46.
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54.
- Pütün, A.E.; Özbay, N.; Önal, E.P.; Pütün, E. Fixed-bed pyrolysis of cotton stalk for liquid and solid products. Fuel Process. Technol. 2005, 86, 1207–1219.
- Primo, A.; Atienzar, P.; Sanchez, E.; Delgado, J.M.; García, H. From biomass wastes to large-area, high-quality, N-doped graphene: Catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 2012, 48, 9254–9256.
- Su, D.S. The Use of Natural Materials in Nanocarbon Synthesis. ChemSusChem 2009, 2, 1009–1020.
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485.
- Marriott, A.; Hunt, A.; Bergström, E.; Wilson, K.; Budarin, V.; Thomas-Oates, J.; Clark, J.; Brydson, R. Investigating the structure of biomass-derived non-graphitizing mesoporous carbons by electron energy loss spectroscopy in the transmission electron microscope and X-ray photoelectron spectroscopy. Carbon 2013, 67, 514–524.
- Titirici, M.-M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116.
- Su, D.S.; Centi, G. A perspective on carbon materials for future energy application. J. Energy Chem. 2013, 22, 151–173.
- Nie, J.-Q.; Zhang, Q.; Zhao, M.-Q.; Huang, J.-Q.; Wen, Q.; Cui, Y.; Qian, W.-Z.; Wei, F. Synthesis of high quality single-walled carbon nanotubes on natural sepiolite and their use for phenol absorption. Carbon 2011, 49, 1568–1580.
- Liu, J.; Deng, Y.; Li, X.; Wang, L. Promising Nitrogen-Rich Porous Carbons Derived from One-Step Calcium Chloride Activation of Biomass-Based Waste for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 177–187.
- El-Hendawy, A.-N.A.; Samra, S.; Girgis, B. Adsorption characteristics of activated carbons obtained from corncobs. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 180, 209–221.
- Zhang, T.; Walawender, W.P.; Fan, L.; Fan, M.; Daugaard, D.; Brown, R. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59.
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005.
- Daifullah, A.A.M.; Yakout, S.M.; Elreefy, S.A. Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. J. Hazard. Mater. 2007, 147, 633–643.
- Aworn, A.; Thiravetyan, P.; Nakbanpote, W. Preparation and characteristics of agricultural waste activated carbon by physical activation having micro- and mesopores. J. Anal. Appl. Pyrolysis 2008, 82, 279–285.
- Balci, S.; Doǧu, T.; Yücel, H. Characterization of activated carbon produced from almond shell and hazelnut shell. J. Chem. Technol. Biotechnol. 1994, 60, 419–426.
- Caturla, F.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Preparation of activated carbon by chemical activation with ZnCl2. Carbon 1991, 29, 999–1007.
- Lillo-Ródenas, M.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275.
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42.
- Hayashi, J.; Horikawa, T.; Takeda, I.; Muroyama, K.; Ani, F.N. Preparing activated carbon from various nutshells by chemical activation with K2CO3. Carbon 2002, 40, 2381–2386.
- US4082694A; Active Carbon Process and Composition. BP Corp North America: Houston, TX, USA, 1978.
- Titirici, M.-M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212.
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822.
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. 2011, 4, 1400–1410.
- Titirici, M.-M.; Thomas, A.; Antonietti, M. Replication and Coating of Silica Templates by Hydrothermal Carbonization. Adv. Funct. Mater. 2007, 17, 1010–1018.
This entry is offline, you can click here to edit this entry!
