The incidence of renal disease is gradually increasing worldwide, and this condition has become a major public health problem because it is a trigger for many other chronic diseases. Cell therapies using multipotent mesenchymal stromal cells, hematopoietic stem cells, macrophages, and other cell types have been used to induce regeneration and provide a cure for acute and chronic kidney disease in experimental models.
- cell therapies
- tissue repair
- kidney disease
- acute kidney injury (AKI)
1. Introduction

2. Multipotent Mesenchymal Stromal Stem Cell Therapies
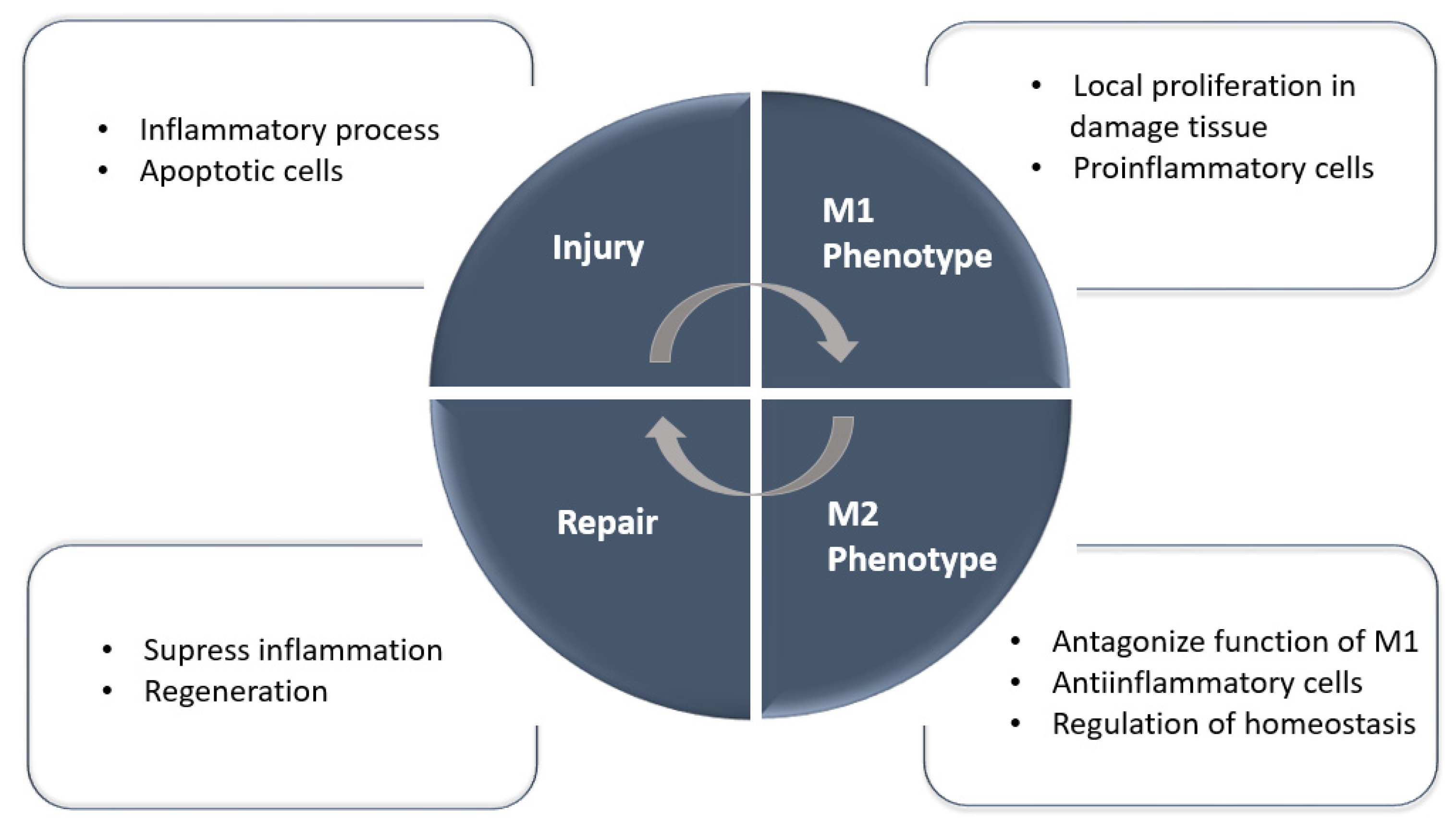
3. Mononuclear and Macrophage Cell Therapies
| Animal Model | Machophage | Genetic Modific (Y/N) |
Treatment | Effects | Year | Ref |
|---|---|---|---|---|---|---|
| BALB/c mice | CD11b+cells isolated from spleen | N | IL-10 1/TGF-β 2 modification |
Significantly attenuated renal inflammation, structural injury and functional | 2010 | [42] |
| FVB/nj mice (Harlan) | Bone marrow | Y | Overexpress HO-1 3 | Preserved renal function and reduced microvascular platelet deposition | 2010 | [43] |
| Sprague–Dawley rat | Bone marrow | Y | Overexpress IL-10 | Decreased the local inflammatory profile and improve renal function | 2012 | [44] |
| Netrin-1 transgenic mice/ C57BL/6J mice | Bone marrow | N | Netrin-1 treated Mac |
Suppressed inflammation and kidney injury | 2013 | [45] |
| C57BL/6 mice | Raw 264.7 | N | MSCs 4 modification |
Supports the transition from tubule injury to tubule repair | 2014 | [46] |
| C57BL/6J mice | Bone marrow | N | IL-4 5/IL-13 6 stimulated | Protected against renal injury and decreased proteinuria | 2016 | [47] |
| C57BL/6J wild- type mice | Bone marrow | N | IL-4/M-CSF 7 stimulated IL-4/IL-13 injection |
Suppressed renal crystal formation | 2016 | [48] |
| Brown Norway rat/Sprague-Dawley rat | Bone marrow | Y | Overexpress LCN-2 8 | Lower susceptibility to ischemic injury | 2016 | [49] |
4. Conclusions
Cellular therapies are among the most exciting innovations in medicine over the last decade and have the potential to offer curative solutions to kidney disease. Overall, there are various preclinical studies that demonstrate the efficacy of different cell therapies, but fewer clinical trials have demonstrated the efficacy of the different cell therapies. The greatest challenge is to understand how to adapt the experimental innovations to a clinical setting and to use appropriate models that link the preclinical assays with the clinical reality in order to apply these therapies to AKI patients. Future directions point to clinical tests with cellular therapies previously proved in preclinical assays and in models near to clinics with no side effects such the described PBMNC therapy [29].
This entry is adapted from the peer-reviewed paper 10.3390/ijms232415943
References
- Tögel, F.E.; Westenfelder, C. Kidney Protection and Regeneration Following Acute Injury: Progress Through Stem Cell Therapy. Am. J. Kidney Dis. 2012, 60, 1012–1022.
- Li, B.; Cohen, A.; Hudson, T.E.; Motlagh, D.; Amrani, D.L.; Duffield, J.S. Mobilized Human Hematopoietic Stem/Progenitor Cells Promote Kidney Repair After Ischemia/Reperfusion Injury. Circulation 2010, 121, 2211–2220.
- de Almeida, D.C.; Donizetti-Oliveira, C.; Barbosa-Costa, P.; Origassa, C.S.; Câmara, N.O. In Search of Mechanisms Associated with Mesenchymal Stem Cell-Based Therapies for Acute Kidney Injury. Clin. Biochem. Rev. 2013, 34, 131.
- Toyohara, T.; Mae, S.-I.; Sueta, S.-I.; Inoue, T.; Yamagishi, Y.; Kawamoto, T.; Kasahara, T.; Hoshina, A.; Toyoda, T.; Tanaka, H.; et al. Cell Therapy Using Human Induced Pluripotent Stem Cell-Derived Renal Progenitors Ameliorates Acute Kidney Injury in Mice. Stem Cells Transl. Med. 2015, 4, 980–992.
- Yuan, X.; Li, D.; Chen, X.; Han, C.; Xu, L.; Huang, T.; Dong, Z.; Zhang, M. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCS) protect against renal ischemia/reperfusion injury via delivering specifity propteis (SP1) and trasxcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017, 8, 3200.
- Wei, X.; Zhang, J.; Gu, Q.; Huang, M.; Zhang, W.; Guo, J.; Zhou, X. Reciprocal Expression of IL-35 and IL-10 Defines Two Distinct Effector Treg Subsets that Are Required for Maintenance of Immune Tolerance. Cell Rep. 2017, 21, 1853–1869.
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507.
- Pan, B.; Fan, G. Stem cell-based treatment of kidney diseases. Exp. Biol. Med. 2020, 245, 902–910.
- Yun, C.; Lee, S. Potential and Therapeutic Efficacy of Cell-based Therapy Using Mesenchymal Stem Cells for Acute/chronic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1619.
- Choi, J.R.; Yong, K.W.; Choi, J.Y. Effects of mechanical loading on human mesenchymal stem cells for cartilage tissue engineering. J. Cell Physiol. 2018, 233, 1913–1928.
- Safwani, W.K.Z.W.; Choi, J.R.; Yong, K.W.; Ting, I.; Adenan, N.A.M.; Pingguan-Murphy, B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology 2017, 75, 91–99.
- Prockop, D.J. Concise Review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells 2013, 31, 2042–2046.
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884.
- Eggenhofer, E.; Luk, F.; Dahlke, M.H.; Hoogduijn, M.J. The Life and Fate of Mesenchymal Stem Cells. Front. Immunol. 2014, 5, 148.
- Tsuchiya, A.; Kojima, Y.; Ikarashi, S.; Seino, S.; Watanabe, Y.; Kawata, Y.; Terai, S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm. Regen. 2017, 37, 16.
- Zhuo, W.; Liao, L.; Xu, T.; Wu, W.; Yang, S.; Tan, J. Mesenchymal Stem Cells Ameliorate Ischemia-Reperfusion-Induced Renal Dysfunction by Improving the Antioxidant/Oxidant Balance in the Ischemic Kidney. Urol. Int. 2011, 86, 191–196.
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect Against Acute Kidney Injury Through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Kidney Blood Press. Res. 2016, 41, 119–128.
- Lee, S.-J.; Ryu, M.-O.; Seo, M.-S.; Park, S.-B.; Ahn, J.-O.; Han, S.-M.; Kang, K.-S.; Bhang, D.-H.; Youn, H.-Y. Mesenchymal Stem Cells Contribute to Improvement of Renal Function in a Canine Kidney Injury Model. In Vivo 2017, 31, 1115–1124.
- Rodrigues, C.E.; Capcha, J.M.C.; de Bragança, A.C.; Sanches, T.R.; Gouveia, P.Q.; de Oliveira, P.A.F.; Malheiros, D.M.A.C.; Volpini, R.A.; Santinho, M.A.R.; Santana, B.A.A.; et al. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res. Ther. 2017, 8, 19.
- Bi, B.; Schmitt, R.; Israilova, M.; Nishio, H.; Cantley, L.G. Stromal Cells Protect against Acute Tubular Injury via an Endocrine Effect. JASN 2007, 18, 2486–2496.
- Perico, N.; Casiraghi, F.; Remuzzi, G. Mesenchymal Stromal Cells for AKI after Cardiac Surgery. JASN 2018, 29, 7–9.
- Swaminathan, M.; Stafford-Smith, M.; Chertow, G.M.; Warnock, D.G.; Paragamian, V.; Brenner, R.M.; Lellouche, F.; Fox-Robichaud, A.; Atta, M.G.; Melby, S.; et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. JASN 2018, 29, 260–267.
- Zhang, M.; Huang, B. The multi-differentiation potential of peripheral blood mononuclear cells. Stem Cell Res. Ther. 2012, 3, 48.
- Mevorach, D.; Zuckerman, T.; Reiner, I.; Shimoni, A.; Samuel, S.; Nagler, A.; Rowe, J.M.; Or, R. Single Infusion of Donor Mononuclear Early Apoptotic Cells as Prophylaxis for Graft-versus-Host Disease in Myeloablative HLA-Matched Allogeneic Bone Marrow Transplantation: A Phase I/IIa Clinical Trial. Biol. Blood Marrow Transplant. 2014, 20, 58–65.
- Yu, S.J.; Yoon, J.-H.; Kim, W.; Lee, J.M.; Bin Lee, Y.; Cho, Y.; Lee, D.H.; Lee, M.; Yoo, J.-J.; Cho, E.J.; et al. Ultrasound-guided percutaneous portal transplantation of peripheral blood monocytes in patients with liver cirrhosis. Korean J. Int. Med. 2017, 32, 261–268.
- Wahid, F.S.A.; Ismail, N.A.; Jamaludin, W.F.W.; Muhamad, N.A.; Idris, M.A.M.; Lai, N.M. Efficacy and Safety of Autologous Cell-based Therapy in Patients with No-option Critical Limb Ischaemia: A Meta-Analysis. Curr. Stem Cell Res. Ther. 2018, 13, 265–283.
- Sermsathanasawadi, N.; Pruekprasert, K.; Chruewkamlow, N.; Kittisares, K.; Warinpong, T.; Chinsakchai, K.; Wongwanit, C.; Ruangsetakit, C.; Mutirangura, P. Peripheral blood mononuclear cell transplantation to treat no-option critical limb ischaemia: Effectiveness and safety. J. Wound Care 2021, 30, 562–567.
- Ohtake, T.; Kobayashi, S.; Slavin, S.; Mochida, Y.; Ishioka, K.; Moriya, H.; Hidaka, S.; Matsuura, R.; Sumida, M.; Katagiri, D.; et al. Human Peripheral Blood Mononuclear Cells Incubated in Vasculogenic Conditioning Medium Dramatically Improve Ischemia/Reperfusion Acute Kidney Injury in Mice. Cell Transpl. 2018, 27, 520–530.
- Játiva, S.; Torrico, S.; Calle, P.; Muñoz, Á.; García, M.; Larque, A.B.; Poch, E.; Hotter, G. NGAL release from peripheral blood mononuclear cells protects against acute kidney injury and prevents AKI induced fibrosis. Biomed. Pharmacother. 2022, 153, 113415.
- Okabe, Y.; Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 2016, 17, 9–17.
- Gosselin, D.; Link, V.M.; Romanoski, C.E.; Fonseca, G.J.; Eichenfield, D.Z.; Spann, N.J.; Stender, J.D.; Chun, H.B.; Garner, H.; Geissmann, F.; et al. Environment Drives Selection and Function of Enhancers Controlling Tissue-Specific Macrophage Identities. Cell 2014, 159, 1327–1340.
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs.Functional Differentiation. Front. Immunol. 2014, 5, 514.
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308.
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223.
- Mittal, M.; Tiruppathi, C.; Nepal, S.; Zhao, Y.-Y.; Grzych, D.; Soni, D.; Prockop, D.J.; Malik, A.B. TNFα-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc. Natl. Acad. Sci. USA 2016, 113, E8151–E8158.
- Chen, T.; Cao, Q.; Wang, Y.; Harris, D.C.H. M2 macrophages in kidney disease: Biology, therapies, and perspectives. Kidney Int. 2019, 95, 760–773.
- Mao, R.; Wang, C.; Zhang, F.; Zhao, M.; Liu, S.; Liao, G.; Li, L.; Chen, Y.; Cheng, J.; Liu, J.; et al. Peritoneal M2 macrophage transplantation as a potential cell therapy for enhancing renal repair in acute kidney injury. J. Cell Mol. Med. 2020, 24, 3314–3327.
- Sheng, J.; Ruedl, C.; Karjalainen, K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity 2015, 43, 382–393.
- Jang, H.-S.; Kim, J.I.; Jung, K.-J.; Kim, J.; Han, K.-H.; Park, K.M. Bone marrow-derived cells play a major role in kidney fibrosis via proliferation and differentiation in the infiltrated site. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 817–825.
- Malone, A.F. Monocytes and Macrophages in Kidney Transplantation and Insights from Single Cell RNA-Seq Studies. Kidney360 2021, 2, 1654–1659.
- Yao, W.; Chen, Y.; Li, Z.; Ji, J.; You, A.; Jin, S.; Ma, Y.; Zhao, Y.; Wang, J.; Qu, L.; et al. Single Cell RNA Sequencing Identifies a Unique Inflammatory Macrophage Subset as a Druggable Target for Alleviating Acute Kidney Injury. Adv. Sci. 2022, 9, 2103675.
- Cao, Q.; Wang, Y.; Zheng, D.; Sun, Y.; Wang, Y.; Lee, V.; Zheng, G.; Tan, T.K.; Ince, J.; Alexander, S.I.; et al. IL-10/TGF-β–Modified Macrophages Induce Regulatory T Cells and Protect against Adriamycin Nephrosis. JASN 2010, 21, 933–942.
- Ferenbach, D.A.; Ramdas, V.; Spencer, N.; Marson, L.; Anegon, I.; Hughes, J.; Kluth, D.C. Macrophages Expressing Heme Oxygenase-1 Improve Renal Function in Ischemia/Reperfusion Injury. Mol. Ther. 2010, 18, 1706–1713.
- Jung, M.; Sola, A.; Hughes, J.; Kluth, D.C.; Vinuesa, E.; Viñas, J.L.; Pérez-Ladaga, A.; Hotter, G. Infusion of IL-10–expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int. 2012, 81, 969–982.
- Ranganathan, P.V.; Jayakumar, C.; Ramesh, G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am. J. Physiol. Ren. Physiol. 2013, 304, F948–F957.
- Geng, Y.; Zhang, L.; Fu, B.; Zhang, J.; Hong, Q.; Hu, J.; Li, D.; Luo, C.; Cui, S.; Zhu, F.; et al. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res. Ther. 2014, 5, 80.
- Du, Q.; Tsuboi, N.; Shi, Y.; Ito, S.; Sugiyama, Y.; Furuhashi, K.; Endo, N.; Kim, H.; Katsuno, T.; Akiyama, S.; et al. Transfusion of CD206+ M2 Macrophages Ameliorates Antibody-Mediated Glomerulonephritis in Mice. Am. J. Pathol. 2016, 186, 3176–3188.
- Taguchi, K.; Okada, A.; Hamamoto, S.; Unno, R.; Moritoki, Y.; Ando, R.; Mizuno, K.; Tozawa, K.; Kohri, K.; Yasui, T. M1/M2-macrophage phenotypes regulate renal calcium oxalate crystal development. Sci. Rep. 2016, 6, 35167.
- Jung, M.; Brüne, B.; Hotter, G.; Sola, A. Macrophage-derived Lipocalin-2 contributes to ischemic resistance mechanisms by protecting from renal injury. Sci. Rep. 2016, 6, 21950.
- Singbartl, K.; Formeck, C.L.; Kellum, J.A. Kidney-Immune System Crosstalk in AKI. Semin. Nephrol. 2019, 39, 96–106.
- Lech, M.; Gröbmayr, R.; Ryu, M.; Lorenz, G.; Hartter, I.; Mulay, S.R.; Susanti, E.; Kobayashi, K.S.; Flavell, R.A.; Anders, H.-J. Macrophage Phenotype Controls Long-Term AKI Outcomes—Kidney Regeneration versus Atrophy. JASN 2014, 25, 292–304.
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic acid: A systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia 2020, 147, 104735.
- Gong, L.; Pan, Q.; Yang, N. Autophagy and Inflammation Regulation in Acute Kidney Injury. Front. Physiol. 2020, 11, 576463.
- Ramanathan, C.; Kathale, N.D.; Liu, D.; Lee, C.; Freeman, D.A.; HogenEsch, J.B.; Cao, R.; Liu, A.C. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018, 14, e1007369.
- Radi, Z.A. Immunopathogenesis of Acute Kidney Injury. Toxicol. Pathol. 2018, 46, 930–943.
- Jia, H.; Yan, Y.; Liang, Z.; Tandra, N.; Zhang, B.; Wang, J.; Xu, W.; Qian, H. Autophagy: A new treatment strategy for MSC-based therapy in acute kidney injury (Review). Mol. Med. Rep. 2017, 17, 3439–3447.
