Many documents indicate that fish may also be a potential source of exposure to chemical pollutants, especially mercury (Hg) (one of the top ten chemicals or groups of chemicals of concern worldwide), and this is a grave concern for many consumers, especially pregnant women, as this could affect their fetuses. Eat fish or not? This is becoming a dilemma for many MOTHERS! This narrative review may help you!
- fish as food
- mercury contamination
- pregnant women
- fetuses
- health impacts
- HgMg
- Environmental Toxicity
- CNS
- BBB
Graphical Abstract:
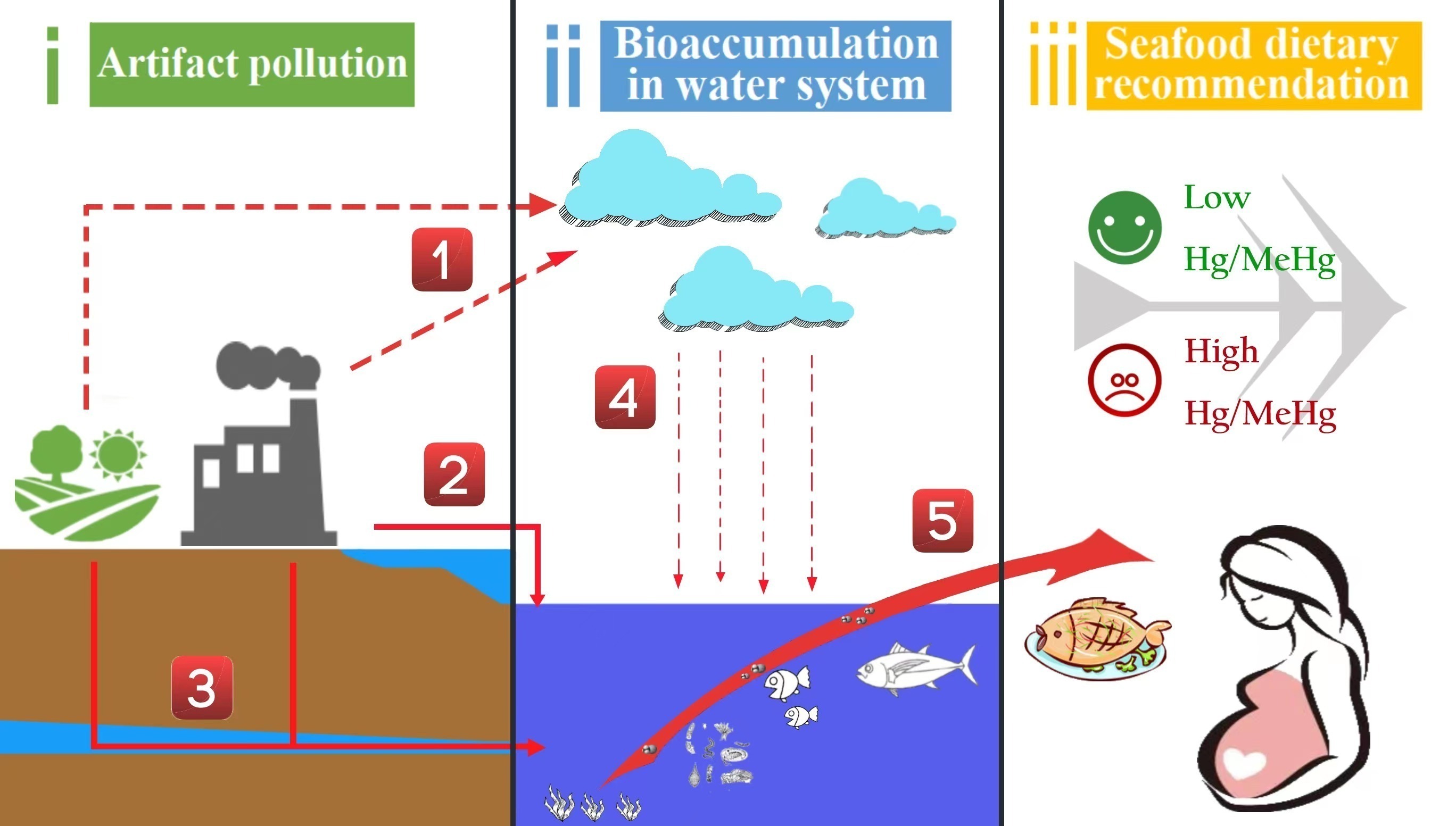
1. Introduction
2. Definition of Mercury (Hg) and Its Forms in Nature and Fish Bodies
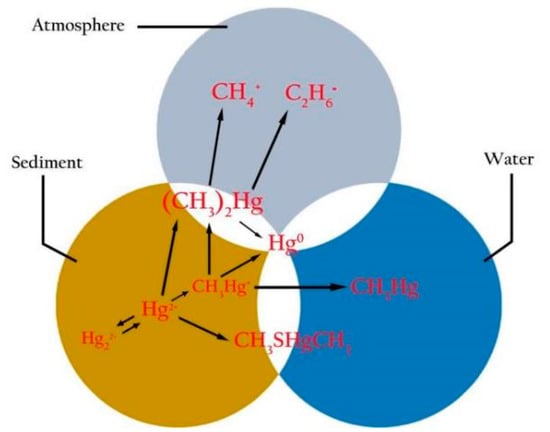
3. Bio-Accumulation, Species-Specific and Geographical Differences in Mercury (Hg) in Fish
3.1. Bio-Accumulation of Hg in Fish through Food Chains
3.2. Species-Specific Difference in Hg in Fish
3.3. Geographical Differences in Hg in Fish
- Toxic effects of mercury (Hg) contaminated fish food on pregnant women and fetuses
4.1. Background of the toxic effects of Hg contamination in fish on fishes and humans
For fishes, Hg markedly affects their physiological health even at lower exposure level, and this is manifested microscopically in the genetic mutations, tissues and physiology, and macroscopically in the survival, growth and developmental status of fish [48]. In addition, Hg exposure can produce teratogenic and neurotoxic effects, and reproductive toxicity, and these effects can then cause harm to cells, tissues, proteins and genes, and ultimately, the survival, growth and behavior of marine fish [48]. There are distinctly individual differences in the factors known to influence the hazard levels of Hg exposure [66]. Thus, there might be non-negligible differences in the effects of Hg toxicity on different individuals, species and life stages of fish [67]. On the other hand, humans can be exposed to Hg, i.e., organic Hg (MeHg/ethyl-Hg) via consumption of fish food and poultry products, and use of insecticides, fungicides and pesticides, in the forms of air pollution, medical equipments (e.g., thermometers and dental amalgam), certain vaccines etc. [68]. The Hg that people usually ingest through feeding fish is organic Hg [25,48]. The most important source of exposure to organic Hg in human beings seems to be the consumption of fish contaminated with MeHg [69]. MeHg bioaccumulates to differing degrees in various fish species and can have serious adverse effects on the development and functioning of the human central nervous system (CNS), especially during prenatal exposure [70]. And the harm of MeHg mainly lies on its toxicity to human nerves, and specifically brain which are most vulnerable to Hg [71]. The World Health Organization (WHO) estimates that the critical blood Hg concentration for MeHg poisoning is 200 μg·L-1 [72]. High level exposure to Hg can result in significantly neurological and behavioral disorders, including tremors, memory loss, neuromuscular changes, renal and thyroid disorders, and even death [73]. And body burden of Hg has been linked to hypertension in populations exposed to high Hg levels, and a significantly positive association between Hg and hypertension, and between Hg and blood pressure (BP) was identified respectively [74].
Hg-toxic effects will differ depending on whether it has been caused by exposure to elemental, inorganic (as salts) or organic Hg compounds, and each form of Hg has a unique toxicological profile and differs in the mechanisms of transport and disposition in human bodies. Exposure to inorganic and organic Hg can lead to the adverse effects including developmental toxicity, immunotoxicity, neurotoxicity and teratogenicity, and especially cytotoxicity, cardiovascular toxicity, hepatotoxicity and nephrotoxicity, and disrupting endocrine systems and metabolic effects for human beings, and all these possible adverse outcomes with Hg exposure may depend on the doses and length of Hg exposure and the Hg forms, and the age and sex of the exposed humans [17-19]. Both MeHg and vapor Hg are highly reactive and interacting mainly with thiol-based-proteins (-SH) in human bodies, and MeHg exerts some toxic effects through altering protease activity, and both metallothioneins and glutathione appear to have a strong relation with the cytotoxicity caused by inorganic and organic Hg, respectively [75]. Moreover, MeHg plays a role affecting several biological processes, including increased lipid peroxidation, reactive oxygen species (ROS) generation and glutathione (GSH) depletion, reduced cell membrane integrity, altered cell signaling and mitochondrial impacts, changed DNA repair and immunomodulatory impacts, affected the regulation of Ca2+, caused glutamate and calcium dyshomeostasis, and changed the DNA methylation etc., which in turn cause adverse effects on humans [17]. Balali-Mood et al. (2021) also indicated that Hg can disrupt cellular events including growth, proliferation, differentiation, damage-repairing processes and apoptosis, and the mechanisms of their action reveal to induce toxicity including ROS generation, weakening of antioxidant defense, enzyme inactivation and oxidative stress, and apoptosis, caspase activation as well as ultrastructural changes in the hepatocytes have also been seen due to Hg exposure [18]. Renu et al. (2021) indicated that Hg can induce apoptosis in liver, and the epigenetic mechanism is that Hg can cause DNA methylation and disruption in the post-transcriptional modifications [19]. And Bridges et al. (2017) indicated that modulation of neurotransmitters including dopamine and serotonin in brain may result in changes of behavior related to Hg exposure [76]. Furthermore, high exposure to Hg can deplete the amount of cellular selenium available for the biosynthesis of thioredoxin reductase and other selenoenzymes that prevent and reverse oxidative damage, which, if the depletion is severe and long-lasting, results in brain cell dysfunctions that can ultimately cause death [77]. Although multiple mechanisms of toxic action of Hg were discussed in [16-19,75], however, many aspects are still far from being understood satisfactorily.
4.2. Toxic effects of Hg contamination in fish food on pregnant women and fetuses
It is undeniable that the intake of fish during pregnancy is beneficial for the body health due to the diverse nutrients it contains. Numerous studies have shown that fish is rich in long chain omega-3 polyunsaturated fatty acids (LCn-3PUFAs), and vitamins A, D and B12, which play an important role in the physiological metabolism [78-79]. However, aquatic organisms, mainly fish, are contaminated with numerous toxic substances, including Hg and other heavy metals and drug residues, which may have adverse effects such as teratogenicity [80-81]. Numerous statements have been made in the medical literature and by WHO in the past, recommending limiting seafood intake by pregnant women to avoid exposure of this population to the potential toxicity of aquatic products [9]. Hg exposure in pregnancy has been associated with both pregnancy complications and developmental problems in infants [82]. In pregnant women, Hg passes through the placental membrane, which can cause spontaneous abortions, premature births, congenital disabilities and retardation of fetus development [83]. And even small amounts of fish consumed by mothers during pregnancy can cause elevated Hg levels and affect children's neurobehavioral development, including basic skills such as listening, reading, and writing [84,85]. Meanwhile, in contrast, no negative effects of maternal fish consumption during pregnancy on local children's neurobehavioral development were found in the Republic of Seychelles [86,87]. However, relevant indicators showed that the population of the Faroe Islands, which consumes mainly whale meat and blubber, contained approximately 10 times more MeHg in their samples at 1.6 ppm than Seychelles fish [87]. The difference in MeHg content undoubtedly contributes to the difference in findings, which also confirms in a comparative way the risk of MeHg in fish for the fetus in pregnant women. Moreover, researchers measured blood Hg levels using atomic absorption based on 200 cases of deliveries in a Chinese hospital, and the incidence of fetal malformations, adverse pregnancy outcomes, hypertensive disorders of pregnancy, intrauterine growth retardation, and fetal distress were found to be higher in the group with elevated blood Hg than in the group with normal blood Hg [88].
For pregnant women, the blood levels of Hg often exceed acceptable international levels, and the average Hg levels in the blood of mothers with premature births, low birthweight and spontaneous abortions were 30% higher in comparison with unexposed women, and a significantly increased risk of premature birth and birth of children with low body weight and spontaneous abortions was also found when the Hg concentration exceeded 2 µg/L of plasma [89]. MeHg is usually absorbed by the body through the skin mucosa, respiratory and digestive tracts, and the most important route is the digestive tract [90]. As a common food item on the human table, the entry of MeHg into the body of pregnant women through the gastro intestinal route is often an important cause of Hg hazards [88]. After entering the bloodstream, Hg binds to the sulfhydryl group of hemoglobin and enters the organs of the body, subsequently the amount of MeHg in the organs and tissues remains relatively constant [91]. It is noteworthy that the toxic effects of MeHg on the liver and kidneys are lower, although the Hg levels in the organs or tissues are in descending order: liver>brain>kidney>blood [90]. And the toxic effects of MeHg in the brain and the nervous system are relatively high [92]. The reason for this is, on the one hand, that brain tissue is rich in lipid-like substances that have a strong affinity for MeHg, which can easily enter brain tissue through blood flow; On the other hand, MeHg is strongly bound to the carbon-Hg chains in the molecular structure of MeHg, so MeHg can remain in brain cells for a long time and cannot be easily excreted [92]. The clearance of MeHg from brain is delayed by 20% compared to other parts of the body, and MeHg accumulation in brain is higher than that in sensory and motor areas, especially in the posterior lobe of the brain [92]. The mechanism of Hg following injury to the mother involves several complex aspects of the body's metabolism. Hg readily binds to sulfhydryl groups and enzymes in proteins leading to dysfunction of several enzymes in the body, including ATPase, lactate dehydrogenase, cytochrome oxidase and alkaline phosphatase, leading to severe enzyme inactivation [91]. Hg can also disrupt the structural integrity of genetic material by binding to multiple groups (e.g., hydroxyl and amino groups) in genetic materials (e.g., DNA and RNA), which can lead to DNA breakage and mutation in severe cases [88]. And Hg exposure often leads to visual field contraction, motor ataxia, dysarthria, tremor and cardiovascular diseases etc. [93,94]. Hg and MeHg can cause mitochondrial dysfunction, decrease ATP synthesis, deplete glutathione, increase phospholipid, protein and DNA peroxidation [95]. The vascular effects of Hg also include many aspects, such as increased oxidative stress and inflammation, and decreased oxidative defenses, mitochondrial dysfunction, depolarization and autoxidation of inner mitochondrial membranes and inactivation of oxygen phosphatase [71].
For the fetus, once incorporated into the body, MeHg easily penetrates the blood-brain barrier (BBB) and causes damage to the central nervous system (CNS) [20]; and high blood Hg levels can increase the incidence of intrauterine growth retardation and fetal distress [96]. In general, Hg can pass placenta into unborn infant, and the early exposure to Hg is correlated to infant health effects, such as neurological, developmental and endocrine disorders [97]. The teratogenicity of MeHg and its effects on fetal growth and development have also been confirmed in a trial of singleton pregnancies [88]. Gilbertson (2004) indicated that the perinatal exposure to MeHg was known to result in severe neurological effects on the developing fetus and infant, including cerebral palsy, mental retardation and seizures [98]. Simultaneously, Hg exposure is also extremely harmful to the fetus, causing cardiovascular disease, hypertension and changes in heart rate variability [99,100]. Since cardiac rhythm and function are controlled by the autonomic nervous system, and it has been hypothesized that the neurotoxic effects of Hg may also affect cardiac autonomic function [101]. Exposure to Hg may have long-term effects on cardiac parasympathetic activity of children, and the intrinsic mechanism why elemental Hg is so damaging to the nervous and cardiovascular systems is due to the high affinity of Hg for sulfhydryl and selenium groups, which are present in glutathione precursors such as cysteine [101].
4.3. Interactive toxic effects of Hg contamination in fish food on pregnant women and fetuses
The main reason for the widespread concern about fish intake by pregnant women is the vulnerability of the pregnant woman and the fetus itself, and the hazard extent of Hg exposure [102]. The fetus is relatively vulnerable to adverse external factors, and the incomplete development of the fetal liver results in the inability to excrete toxic substances and pollutants in a timely and effective manner [103]. The dangers of Hg are also passed between the pregnant woman and the fetus and affect each other [102]. On the one hand, the pregnant women who consumed a large amount of fish might have elevated blood Hg levels, and Hg level in cord blood is much higher than that in maternal blood [9]. Kim & Kim (2006) also indicated that the blood Hg content in the umbilical blood is substantially higher, and this may lead to higher blood Hg levels in neonates [9]. Higher Hg concentration in the fetus compared to that in the mother may affect immature fetal organs [104]. And the placenta in pregnant women does not present a barrier to Hg and the fetus has a high accumulation capacity for MeHg with 1.8 - 4.0 times of MeHg content in brain, liver, kidney, heart or lung than that of normal adults [105]. On the other hand, MeHg has lipolytic properties with a strong affinity for lipid-like substances and can easily pass through membranous tissues, such as the placental barrier and the blood-brain barrier (BBB), and it is easy to cause direct all-round damage to the fetus [20]. Because of its lipid solubility and short-chain hydrocarbon structure, Hg can rapidly pass through the placenta and be oxidized into ionic complexes that bind with high affinity to fetal hemoglobin and cannot be returned to the pregnant women's blood circulation [38,83]. Moreover, Hg easily binds to sulfhydryl groups, so proteins and enzymes containing sulfhydryl groups (e.g., ATPase and lactate dehydrogenase) are disturbed or even inactivated by the binding of Hg [91]. And genetic material containing amino and phosphate groups (e.g., DNA) can be damaged by Hg binding [88].
Based on the toxic effects of Hg contaminated fish food on pregnant women and fetuses, and in combination with the reviewed multiple mechanisms of Hg-toxic action on humans [16-19,75], the action model of high exposure to Hg for pregnant women and fetuses through consumption of Hg contaminated fish food was elucidated in Figure 2, which was adapted from Balali-Mood et al. (2021) [18]. It is elucidated that high exposure to Hg for pregnant women and fetuses is harmful and has many adverse effects on various organs (especially liver, kidney and brain), and disruption of the antioxidant system may play an important role in the Hg toxic effects, simultaneously signaling transduction, protein or/and enzyme activity, and gene regulation are involved in mediating toxic and adaptive response to Hg exposure. The information of mechanism involved in Hg toxicity is growing, but knowledge gaps still exist between the adverse effects and mechanisms of action, especially at the molecular level.
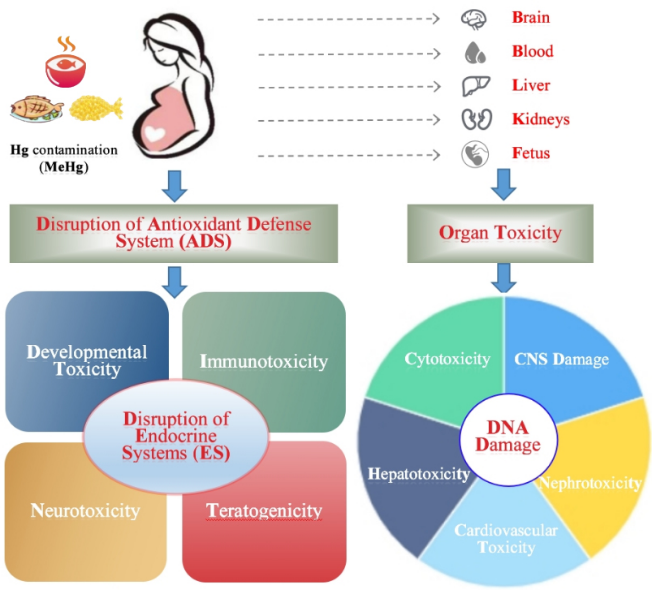
Figure 2. Action model of high exposure to mercury (Hg) for pregnant women and fetuses through consumption of Hg contaminated fish food (Note: ADS-Antioxidant Defense System (including various enzymatic and nonenzymatic antioxidants); ES-Endocrine Systems (i.e., Glands) produce and release different hormones; CNS-Central Nervous System, and Hg can inhibit the formation of myelin to prevent nerve sheaths from forming properly; Blood and brain-Forming the blood-brain barrier (BBB) against toxic chemicals, and MeHg easily penetrates BBB and causes CNS damage particularly in fetuses; Liver-Hg can induce apoptosis in liver through DNA damage with disrupting DNA methylation and disruption in the post-transcriptional modifications. Adapted from Balali-Mood et al. (2021) [18])
- Recommended fish diet for pregnant women based on toxic effects of Hg contamination in fish food
Fish is considered as a healthy food with exceptional properties rich in vitamins, minerals and high quality proteins and essential 3FAs [1-5]. During pregnancy period, pregnant women need more nutritional supplements than ever before. However, given the toxic effects of Hg contained in fish products, there is often a “trade-off” for them in fish consumption to achieve a relatively favorable ratio [6,104,106-107]. The rational consumption of fish is a mustin terms of nutrient intake, ensuring the health safety and concerns of pregnant women [11,108]. On the one hand, more innovative and new fish products need to be developed to better suit the seafood intake needs of pregnant women. On the other hand, the proper understanding of fish consumption needs to be further promoted and popularized in general, especially for pregnant women. Some researchers recommend regular fish oil supplementation during pregnancy [109]. Although many reports elucidated the benefits of fish oil [110-111], some studies have concluded that it is not beneficial or have even reached the opposite conclusion [112-114]. Given the effects of high doses of cod liver oil on hypertension in pregnancy [113] and some adverse effects of MeHg arising from the consumption of regular fish oil [115], the rational recommendation of dietary fish intake is a must due to the complex interplay between MeHg and fish oil-derived fatty acids [116]. Fish oils come from different fish species and involve some variation in the contamination status and purification level of the origin, and therefore the most conservative recommendation is to consume a variety of low Hg contaminated fish food for health benefits [117].
Additionally, some researchers recommend eating small fish because they have low levels of bio-concentration of toxic substances such as Hg in their bodies [44,57]. And avoiding specific species (mainly carnivorous fish), limiting the intake of fish for pregnant women and avoiding intake of fish that survive from heavily polluted waters, and selective intake of aquatic products (e.g., shellfish and shrimp) is also recommended. The importance of fish food should not be overlooked because of the toxic effects of harmful substances (including Hg), and the benefits of important nutrients need to be properly and widely disseminated to the consumer community, especially pregnant women. In a study on the effects of fish consumption and fetal neurodevelopment in women of childbearing age, the authors stated that fish consumption in dietary intake should focus on nutrients, such as docosahexenoic acid (DHA) as mentioned by Mendivil (2021) [4]. The population of pregnant women is encouraged to consume fish with high DHA and low MeHg, such as anchovy, arctic char, atlantic mackerel, catfish, cod, haddock, herring, perch, pollock, salmon, sardines, shellfish, tilapia, trout and tuna etc., and strictly avoid consuming fish with high MeHg levels (such as bluefish, croaker, eel, king mackerel, shark, swordfish, tilefish and weakfish etc.) (as described in Table 1).
Table 1. Choice guidelines of the species and types of fish based on the Hg contamination, as daily fish food for pregnant women.
|
Choices |
Level of Hg contamination |
Types of fish |
Species of fish |
Cited references |
|
Right |
Low Hg/MeHg |
Freshwater fish, herbivorous fish, small fish etc. |
Anchovy, arctic char, atlantic mackerel, catfish, cod, haddock, herring, perch, pollock, salmon, sardines, shellfish, tilapia, trout and tuna etc. |
42-47, 49- 53, 107, 118, 119 |
|
Wrong |
High Hg/MeHg |
Marine fish, piscivores and carnivores, benthic fishes, large fish, predatory fish etc. |
Bluefish, croaker, eel, king mackerel, shark, swordfish, tilefish and weakfish etc. |
As for the frequency and amount of fish consumption, the relevant safety and health authorities have given different recommendations based on different countries or regions. For instance, in March 2004, the Department of Health and Human Services (HHS) and the Environment Protection Agency (EPA) of the USA published a report entitled “What You Need to Know about Mercury in Fish and Shellfish" which showed that nearly all fish and shellfish contain trace amounts of Hg. Thus, to avoid Hg contamination, in 2010, the U.S. Department of Agriculture (USDA) and the U.S. Department of HHS recommended the public to consume not more than 8 ounces (227 g) of a variety of seafood per week, which equates to an average daily intake of 250 mg of fatty acids, including DHA [120]. In addition, the US EPA recommended that the total blood Hg concentration should remain lower than 5.8 µg/L for women of childbearing age [121]. The British Food Standard Agency (FSA) recommended that pregnant women, women of childbearing age and children under 16 years of age should avoid consuming swordfish and tuna (which are higher ranking in ocean food chain) because of the high Hg content and recommended that pregnant women and women of childbearing age should avoid consuming more than two tuna steak per week [72, 122-123].
Many documents have also presented fish consumption views and guidelines for pregnant women [6,52,106]. However, these recommendations tend to be generalized in nature. Specifically, for individuals, the amount of fish recommended per week depends on the frequency and portion size of fish a person eats, the individual's physical condition such as the individual's sensitivity to toxicity, his or her own body weight, etc. More precise recommendations can be obtained from national or local public health departments [118]. In addition, based upon the study on the awareness of fish Hg and in compliance with WHO’s enforcement of fish consumption among pregnant women, researchers found that women with higher incomes and education and those living in coastal states were more likely to be aware of Hg in fish food, suggesting that information about safe fish consumption is not being communicated equally to all groups [124-125].
However, there have been many studies on the toxicity of MeHg in fish, which have drawn opposite conclusions. The risk of Hg content caused by moderate fish intake is less than the beneficial effects of fish nutrients on human body, and they maintain a positive attitude towards fish intake as a whole [126]. Gale et al. (2008) indicated that as oily fish is a major dietary source of 3FAs, it is possible that low intake of fish during pregnancy may have adverse effects on the developing fetal brain [127]. And many experiments have shown that Hg does not affect pregnant women and fetuses in small amounts [10,108,128-129]. No significant conversion of Hg species was also observed after fish cooking treatment, while an overall loss of up to 33% of Hg species in fish was observed after frying and most of the Hg lost during the cooking procedure came from CH3Hg+, so it was concluded that the fish diet was neutral especially after cooking treatment [130]. There is no consensus on the effects of fish containing MeHg on pregnant women and the neurological effects on the fetus. Persistent chemical pollutants may bio-accumulate and have the potential to achieve teratogenic or other adverse effects. In addition, the ultimate consequences of exposure to toxic chemicals (including Hg) in pregnant women, especially in the long term, are uncertain [131]. Therefore, some studies concluding that exposure to low levels of chemical toxins (including Hg) during pregnancy has no long-term effects are incomplete and inappropriate, and more high quality precise and scientific researchers are warranted.to further carry out in the future.
The researchers hitherto found low-Hg dietary intake of fish per week, where almost all women consume less than 6 ounces which is equivalent to WHO’s recommendation of 170.1 g as per week, is the current existing scenario, however the average fish intake for the female population ranged from 89 g to 120 g (2 to 3 ounces) per week or less. The overall fish consumption pattern of females is followed as pregnant women > postpartum women > normal group of women [128]. This finding is similar to the FDA’s analysis of fish consumption, which estimated that average fish consumption of all women aged (16-45) to be 13.4 g per day (i.e., 93.8 g per week), and the 2003-2004 NHANES results estimated that women aged 16-45 years averaged 10.3 g fish per day [132]. These data respond to a phenomenon that indicates a deficit in the promotion of fish consumption. The predominantly pregnant and postpartum women appear to be following national or local safety and health organization recommendations of "no eating" rather than maintaining a regular intake of low-Hg fish, this results in pregnant women not consuming enough low-Hg fish to benefit for their health [125,128]. Therefore, education and media coverage for pregnant women needs to be further improved, and the government and society need to take measures to avoid extreme attitudes toward fish consumption among pregnant women, i.e., total ban or no concern at all. Agencies need to adequately communicate the benefits of consuming adequate amounts of low-Hg fish food while in the meantime raising awareness among pregnant women about the dangers of Hg contamination in fish and other fish products, as per described in this review. Moreover, both metallothioneins and glutathione appear to have a strong relation with inorganic and organic Hg cytotoxicity respectively [75]. And hair is considered as an index of Hg exposure since MeHg is accumulated there (the average ratio of hair to blood concentrations of MeHg is about 250:1) and Hg is excreted in urine and feces [76]. So the hair, urine and feces, and some specific proteins of pregnant women can be used as special biomarkers as early warning of Hg contamination for the daily intake of fish in the diet.
- Conclusion and perspectives
As one of the top ten chemicals or groups of chemicals of major public health concern, Hg has been continuously discharged from natural sources and industrial activities, and the health effects of Hg contamination on humans, especially pregnant women (including fetuses) susceptible to Hg (especially MeHg) exposure even though at low levels become a worldwide concern. This review paper introduced the Hg forms in the nature and fish bodies, and the bio-accumulation of Hg in fish through food chains in water ecosystem, and further reviewed the interactive toxic effects and action mechanisms of Hg-contaminated fish food on pregnant women and fetuses. Based on the mechanisms that inorganic Hg cannot pass the blood brain barrier (BBB) and placenta, and liquid Hg0 is just slightly absorbed from the gastrointestinal (GI) tract, so these two forms of Hg does not appear to be toxic as accidentally contacting small amounts, while vapor Hg0 and organic Hg (including MeHg) have high toxic even at low levels because they can pass the BBB (blood-brain barrier) and cause CNS (central nervous system) disorder. Because there are species-specific and geographical difference of Hg bio-accumulation in fish, this review paper provides practical recommendations for people, especially pregnant women to select right species and specific tissues of fish and seafood with low concentrations of Hg in fish and suggest cooking fish diet. In this review paper, it was addressed this important public health dilemma for pregnant women to eat or not eat fish exposed to mixtures of healthful nutrients and Hg contamination. In the future, based on the accurate measurement of the Hg content in different species of fish in the corresponding waters of different regions and the detailed classification of different populations of Hg-contaminated fish in the region, the local governments and health organizations should further provide more accurate and personalized fish dietary intake recommendations for specific populations of childbearing-age women who might become pregnant, are pregnant, and nursing mothers, and young children under 16 years of age, so as to ensure the maximum benefits of fish dietary intake.
References
- Mohanty, B.P.; Mahanty, A.;Ganguly, S.; Mitra, T.; Karunakaran, D.; Anandan, R. Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem2019, 293, 561-570.
- Shehzad, A.; Zahid, A.; Latif, A.; Amir, R.M.; Suleria, H.A.R. Marine foods: nutritional significance and their industrial applications. In: Goyal, M.R.; Suleria, H.A.R.; Kirubanandan, S. (eds). Technological Processes for Marine Foods, From Water to Fork. New York: Apple Academic Press, 2019, pp. 289-306.
- Pyz-Łukasik, R.; Chałabis-Mazurek, A.; Gondek, M. Basic and functional nutrients in the muscles of fish: a review. J. Food. Prop2020, 23 (1), 1941-1950.
- Mendivil, C.O. Dietary fish, fish nutrients, and immune function: a review. Nutr2021, 7, 617652.
- Mendivil, C.O. Fish consumption: a review of its effects on metabolic and hormonal health. Metab. Insights2021, 14, 1-6.
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA2006, 296, 1885-1899.
- Bloomingdale, A.; Guthrie, L.B.; Price, S.; Wright, R.O.; Platek, D.; Haines, J.; Oken. A qualitative study of fish consumption during pregnancy. J. Clin. Nutr2010, 92 (5), 1234-1240.
- Elsayed, H.; Yigiterhan, O.; Al-Ansari, M.; Al-Ashwel, A.A.; Elezz, A.A.; Al-Maslamani, I.A. Methylmercury bioaccumulation among different food chain levels in the EEZ of Qatar (Arabian Gulf). Reg. Stud. Mar. Sci2020, 37, 101334.
- Kim, E.H.; Kim, I.K. The effect of fish consumption on blood mercury levels of pregnant women, Yonsei Med. J2006, 47 (5), 626-633.
- Hibbeln, J.R.; Davis, J.M.; Steer C, Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study.Lancet 2007, 369, 578-585.
- Taylor, A.L.; Collins, C.E.; Patterson, A.J. The relationship between potential contaminant exposure from fish and nutrient intakes in Australian women by pregnancy status.Nutr. Diet 2014, 71 (4), 229-235.
- Starling, P.; Charlton, K.; McMahon, A.T.; Lucas, C. Fish intake during pregnancy and foetalneurodevelopment-A systematic review of the evidence. Nutrients 2015, 7, 2001-2014.
- Lucas, C.; Starling, P.; McMahon, A.; Charlton, K. Erring on the side of caution: pregnant women's perceptions of consuming fish in a risk averse society. Hum. Nutr. Diet2016, 29 (4), 418-426.
- Shalini, R.; Jeyasekaran, G.; Shakila, R.J.; Sundhar, S.; Arisekar, U.; Jawahar, P.; Aanand, S.; Sivaraman, B.; Malini, A.H.; Surya, T. Dietary intake of trace elements from commercially important fish and shellfish of Thoothukudialong the southeast coast of India and implications for human health risk assessment. Pollut. Bull 2021, 173, 113020.
- US Environment Protection Agency (US EPA). Mercury Study Report to Congress. EPA-452/R-97. Washington, DC: Office of Research and Development. 1997.
- Rice, K.M.; Walker, E.M.; Jr, Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. Prev. Med. Public Health2014, 47 (2), 74-83.
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: molecular evidence. Chemosphere2020, 245, 125586.
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Pharmacol. 2021, 12, 643972.
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Gopalakrishnan, A.V. Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium) - induced hepatotoxicity: A review. Chemosphere2021, 271, 129735.
- Díez, S. Human health effects of methylmercury exposure. Environ. Contam. Toxicol2009, 198, 111-132.
- Zhang, L.; Wong, M.H. Environmental mercury contamination in China: Sources and impacts. Int2007, 33, 108-121.
- Bjørklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The toxicology of mercury: Current research and emerging trends. Res2017, 159, 545‐554.
- World Health Organization (WHO). Mercury and Health. 2017. <https://www.who.int/news-room/fact-sheets/detail/mercury-and-health>
- Sakamoto, M.; Nakamura, M.; Murata, K. Mercury as a global pollutant and mercury exposure assessment and health effects. Nihon EiseigakuZasshi 2018, 73 (3), 258-264.
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The causes and effects of mercury and methylmercury contamination in the marine environment: A review. Curr. Pollut. Rep2022, 8, 249-272.
- Kungolos, A.; Aoyama, I.; Muramoto, S. Toxicity of organic and inorganic mercury to Saccharomyces cerevisiae. Ecotoxicol. Environ. Saf1999, 43 (2), 149–155.
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Enriron. Sci. Eur2020, 32, 128.
- Finley, M.L.; Kidd, K.A.; Curry, R.A.; Lescord, G.L.; Clayden, M,G,; O’Driscoll, N,J. A comparison of mercury biomagnification through lacustrine food webs supporting brook trout (Salvelinus fontinalis) and other salmonid fishes. Environ. Sci2016, 4, 23.
- Hrabik, T.R.; Watras, C.J. Recent declines in mercury concentration in a freshwater fishery: isolating the effects of de-acidification and decreased atmospheric mercury deposition in Little Rock Lake, Total Environ2002, 297, 229-237.
- Davis, J.A.; Looker, R.E.; Yee, D.; Pasquale, M.M.D.; Grenier, J.L.; Austin, C.M.; McKee, L.J.; Greenfield, B.K.; Brodberg, R.K.; Blum, J.D. Reducing methylmercury accumulation in the food webs of San Francisco Bay and its local watersheds. Res2012, 119, 3-26.
- Lavoie, R.A.; Jardine, T.D.; Chumcha, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Sci. Technol2013, 47, 13385−13394.
- Harris, R.C.; Rudd, J.W.M.; Amyot, M.; Babiarz, C.L.; Beaty, K.G.; Blanchfield, P.J.; Bodaly, R.A.; Branfireun, B.A.; Gilmour, C.C.; Graydon, J.A.; Heyes, A.; Hintelmann, H.; Hurley, J.P.; Kelly, C.A.; Krabbenhoft, D.P.; Lindberg, S.E.; Mason, R.P.; Paterson, M.J.; Podemski, C.L.; Robinson, A.; Sandilands, K.A.; Southworth, G.R.; St Louis, V.L.; TateetT. Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition. PNAS2007, 104 (42), 16586-16591.
- Regnell, O.; Watras, C.J. Microbial mercury methylation in aquatic environments: A critical review of published field and laboratory studies. Sci. Technol2018, 53 (1), 4-19.
- Wood, J.F.; Kennedy, F.S.; Rosen, C.G. Synthesis of methylmercury compounds by extractsofmethanogenic bacterium. Nature 1968, 220, 173-174.
- Fu, D.Y.; Wang, S.H.; Qi, S.H. Environmental factors affecting the biomethylation of mercury. China Environ. Sci1982, 4, 49-54.
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of methylmercury in the environment on wild birds, mammals and fish, Ambio2007, 36, 11-17.
- Wu, P.; Kainz, M.J.; Bravo, A.G.; Åkerblom, S.; Sonesten, L.; Bishop, K. The importance of bioconcentration into the pelagic food web base for methylmercury biomagnification: A meta-analysis. Total Environ2019, 646, 357-367.
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Rev. Toxicol2006, 36, 609-662.
- Afandi, I.; Talba, S.; Benhra, A.; Benbrahim, S.; Chfiri, R.; Labonne, M.; Masski, H.; Laë, R.; Tito, L.; De Morais, L.T.;Bekkali, M.; Bouthir, F.Z. Trace metal distribution in pelagic fish species from the north-west African coast (Morocco). Aquat. Res 2018, 10 (2), 191-205.
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in farmed and wild atlanticbluefin tuna (Thunnus thynnus) muscle. Molecules 2019, 24 (7), 1273.
- Watras, C.J.; Back, R.C.; Halvorsen, S.; Hudson, R.J.M. Bioaccumulation of mercury in pelagic freshwater food webs. Total Environ1998, 219, 183–208.
- Kojadinovic, J.; Potier, M.; Corre, M.L.; Cosson, R.P.; Bustamantec, P. Mercury content in commercial pelagic fish and its risk assessment in the Western Indian Ocean. Total Environ2006, 366, 688-700.
- Wang, X.; Wang, W.X. The three ‘B’ of fish mercury in china: bioaccumulation, biodynamics and biotransformation. Pollut2019, 250, 216-232.
- Backstrom, C.H.; Buckman, K.; Molden, E.; Chen, C.Y. Mercury levels in freshwater fish: Estimating concentration with fish length to determine exposures through fish consumption. Environ. Contam. Toxicol2020, 78 (4), 604-621.
- Davis, J.A.; Ross, J.R.M.; Bezalel, S.; Sim, L.; Bonnema, A.; Ichikawa, G.; Heim, W.A.; Schiff, K.; Eagles-Smith, C.A.; Ackerman, J.T. Hg concentrations in fish from coastal waters of California and Western North America. Total Environ2016, 568, 1146-1156.
- da Silva, S.F.; Pereira, J.P.G.; Oliveira, D.C.; de Oliveira Lima, M. Methylmercury in predatory and non-predatory fish species marketed in the Amazon Triple Frontier. Environ. Contam. Toxicol2020, 104 (6), 733-737.
- Nyholt, K.; Jardine, T.D.; Villamarín, F.; Jacobi, C.M.; Hawes, J.E.; Campos-Silva, J.V.; Srayko, S.; Magnusson, W.E. High rates of mercury biomagnificationin fish from Amazonian floodplain-lake food webs. Total Environ 2022, 833, 155161.
- Zheng, N.; Wang, S.J.; Dong, W.; Hua, X.Y.; Li, Y.Y.; Song, X..; Chu, Q.W.; Hou, S.N.; Li, Y. The toxicological effects of mercury exposure in marine fish. Environ. Contam. Toxicol2019, 102 (5), 714-720.
- Parang, H.; Esmaeilbeigi, M. Total mercury concentration in the muscle of four mostly consumed fish and associated human health risks for fishermen and non-fishermen families in the AnzaliWetland, Southern Caspian Sea. Stud. Mar. Sci 2022, 52, 102270.
- Buck, D.G.; Evers, D.C.; Adams, E.; DiGangi, J.; Beeler, B.; Samánekm; PetrlikmJ.; Turnquist, M.A.; Speranskaya, O.; Regan, K.; Johnson, S. A global-scale assessment of fish mercury concentrations and the identification of biological hotspots. Sci. Total Environ 2019, 687, 956-966.
- Zou ,C.X.; Yin, D.Q.; Wang, R. Mercury and selenium bioaccumulation in wild commercial fish in the coastal East China Sea: Selenium benefits versus mercury risks. Pollut. Bull2022, 180, 113754.
- Burger, J.; Gochfeld, M.A. Risk to consumers from mercury in Pacific cod (Gadus macrocephalus) from the Aleutians: Fish age and size effects. Res2007, 105, 276-284.
- Vieira, H.C.; Ramirez, M.M.B.; Bordalo, M.D.; Rodrigues, A.C.M.; Soares, A.M.V.M.; Abreu, S.N.; Morgado, F.; Rendón-von Osten, J.R. Totaland organic mercury in fish from different geographical areas in the North Atlantic Ocean and health risk assessment. Health 2021, 13, 361-373.
- Jackson, T.A. Biological and environmental control of mercury accumulation by fish in lakes and reservoirs of Northern Manitoba, Canada. J. Fish Aquat. Sci1991, 48, 2449–2470.
- Wiener, J.G.; Krabbenhoft, D.P.; Heinz, G.H.; Scheuhammer, M. Ecotoxicology of Mercury. In: Hoffman DJ, Rattner BA, Burton Jr GA, Cairns Jr J (Eds.). Handbook of Ecotoxicology. Lewis Publications, Boca Raton, FL, 2003, pp. 409–463.
- Costa, F.; Coelho, J.P.; Baptista, J.; Martinho, F.; Pereira, M.E.; Pardal, M.A. Mercury accumulation in fish species along the Portuguese coast: Are there potential risks to human health? Pollut. Bull2020, 150, 110740.
- Dang, F.; Wang, W.X. Why mercury concentration increases with fish size? Pollut2012, 163, 192-198.
- Li, W.Z.; Wang, W.X. Inter-species differences of total mercury and methylmercury in farmed fish in Southern China: Does feed matter? Total Environ2019, 651, 1857-1866.
- Madenjian, C.P.; Rediske, R.R.; Krabbenhoft, D.P.; Stapanian, M.A.; Chernyak, S.M.; O’Keefe, J.P. Sex differences in contaminant concentrations of fish: a synthesis. Sex Differ2016, 7, 42.
- Renzoni,; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res1998, 77, 68-72.
- Mok, W.J.; Seoka, M.; Tsukamasa, Y.; Kawasaki, K.; Ando, M. Mercury levels of small fishes: influence of size and catch area. Fis Sci2011, 77, 823.
- Depew, D.C.; Burgess, N.M.; Anderson, M.; Baker, R.; Bhavsar, S.P.; Bodaly, R.A.; Eckley, C.S.; Evans, M.S.; Gantner, N.; Graydon, J.A.; Jacobs, K.; Leblanc, J.E.; St Louis, V.L.; Campbell, L.M. An overview of mercury concentrations in freshwater fish species: a national fish mercury dataset for Canada. J. Fish. Aquat. Sci2013, 70, 436-451.
- Llull, R.M.; Gari, M.; Canals, M.; Rey-Maquieira, T.; Grimalt, J. Mercury concentrations in lean fish from the Western Mediterranean Sea: Dietary exposure and risk assessment in the population of the Balearic Islands. Environ. Res2017, 158, 16-23.
- Bank, M.S.; Chesney, E.; Shine, J.P.; Maage, A.; Senn, D.B. Mercury bioaccumulation and trophic transfer in sympatric snapper species from the Gulf of Mexico. Appl 2007, 17, 2100-2110.
- Piraino, M.N.; Taylor, D.L. Bioaccumulation and trophic transfer of mercury in striped bass (Morone saxatilis) and tautog (Tautoga onitis) from the Narragansett Bay (Rhode Island, USA). Environ. Res2009, 67, 117-128.
- Zhang, Q.F.; Li, Y.W.; Liu, Z.H.; Chen, Q.L. Reproductive toxicity of inorganic mercury exposure in adult zebrafish: Histological damage, oxidative stress, and alterations of sex hormone and gene expression in the hypothalamc-pituitary-gonadal axis. Toxicol2016, 177, 417–424.
- Morcillo, P.; Esteban, M.A.; Cuesta, A. Mercury and its toxic effects on fish. AIMS Environ. Sci2017, 4 (3), 386-402..
- Crowe, W.; Allsopp, P.J.; Watson, G.E.; Magee, P.; Strain, J.J.; Armstrong, D.J.; Ball, E.; McSorley, E.M. Mercury as an environmental stimulus in the development of autoimmunity-A systematic review. Rev2017, 16 (1), 72-80.
- Kimáková, T.; Kuzmová, L.; Nevolná, Z.; Bencko, V. Fish and fish products as risk factors of mercury exposure. Agr. Env. Med2018, 25, 488‐493.
- Morrissette, J.; Takser, L.; St-Amour, G.; Smargiassi, A.; Lafond, J.; Mergler, D. Temporal variation of blood and hair mercury levels in pregnancy in relation to fish consumption history in a population living along the St. Lawrence River. Res2004, 95 (3), 363-734.
- Takahashi, T.; Shimohata, T. Vascular dysfunction induced by mercury exposure. J. Mol. Sci2019, 20 (10), 2435.
- World Health Organization (WHO). Methylmercury - Environmental Health Criteria 101. 1990. <https://wedocs.unep.org/handle/20.500.11822/29413>
- Karagas, M.R.; Choi, A.L.; Oken, E.; Horvat, M.; Schoeny, R.; Kamai, E.; Cowell, W.; Grandjean, P.; Korrick, S. Evidence on the human health effects of low level methylmercury exposure. Health Perspect2012, 120, 799-806.
- Hu, X.F.; Singh, K.; Chan, H.M. Mercury exposure, blood pressure, and hypertension: A systematic review and dose-response meta-analysis. Health Perspect2018, 126 (7), 076002.
- Guzzi, G.P.; La Porta, C.A.M. Molecular mechanisms triggered by mercury. Toxicology2008, 244, 1-12.
- Bridges, C.C.; Zalups, R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Toxicol2017, 91 (1), 63-81.
- Wikipedia Mechanism (Creative Commons). Mercury Toxicity - Mechanism. 2022. <https://www.liquisearch.com/mercury_toxicity/mechanism>
- Simpson, J.L.; Bailey, L.B.; Pietrzik, K.; Shane, B.; Holzgreve, W. Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part I – Folate, vitamin B12, vitamin B6. Matern.-Fetal Neonatal. Med2010, 23, 1323-1343.
- Simpson, J.L.; Bailey, L.B.; Pietrzik, K.; Shane, B.; Holzgreve, W. Micronutrients and women of reproductive potential: Required dietary intake and consequences of dietary deficienty or excess. Part II—Vitamin D, vitamin A, Iron, Zinc, Iodine, Essential Fatty Acids, Matern.-Fetal Neonatal. Med2011, 24, 1–24.
- Bjerregaard, P.; Hansen, J.C. Organochlorines and heavy metals in pregnant women from the Disko Bay area in Greenland. Total Environ, 2000, 245, 195–202.
- Olsen, S.F. Commentary: Mercury, PCB, and now eicosapentaenoic acid: Still another reason why pregnant women should be concerned about eating seafood? J. Epidemiol2001, 30, 1279–1280.
- Solan, T.D.; Lindow, S.W. Mercury exposure in pregnancy: A review. Perinat. Med2014, 42 (6), 725-729.
- Bjørklund, G.; Chirumbolo, S.; Dadar, M.; Pivina, L.; Lindh, U.; Butnariu, M.; Aaseth, J. Mercury exposure and its effects on fertility and pregnancy outcome. Basic Clin. Pharmacol. Toxicol2019, 125, 317–327.
- Grandjean, P.; Weihe, P.; White, R.F.; Debes, F.; Araki, S.; Yokoyama, K.; Murata, K.; SØRENSEN, N.; Dahl, R.; JJØRGENSEN P. 1997. Cognitive deficit in 7-year-old children with prenatal exposure to methyl mercury. Teratol1997, 19, 417-428.
- Steuerwald, U.;‚Weihe, P.;‚Jorgensen, P.J.;‚Bjerve, K.; Brock, J.; Heinzow, B.; Budtz-Jørgensen, E.; Grandjean, P. Maternal seafood diet methylmercury exposure‚and neonatal neurologic function. Pediatr2000, 136, 599-605.
- Davidson, P.W.;‚Myers, G.J.;‚Cox, C.; Axtell, C.; Shamlaye, C.; Sloane-Reeves, J.; Cernichiari, E.; Needham, L.; Choi, A.; Wang, Y.; Berlin, B.; Clarkson, T.W. Effects of prenatal and postnatal methymercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA1998, 280, 701-707.
- Palumbo, D.R.;‚Cox, C.;‚Davidson, P.W.; Myers, G.W.; Choi, A.; Shamlaye, C.; Sloane-Reeves, J.; Cernichiar,i E.; Clarkson, T.W. Association between prenatal exposure to methylmercury and cognitive functioning in Seychellois children: A reanalysis of the McCarthy Scales of Children’s Ability from the main corhort study. Res‚2000, 84, 81-88.
- Zhu, H.; Yang, Z. Effects of mercury exposure during pregnancy on pregnancy outcome and related factors. Hainan Med. J2010, 21, 25-28.
- Dudarev, A.; Odland, J.O.; Reiersen, L.O. The Russian arctic mother‐child cohort—the first results of a follow up study of persistent toxic substances (PTS) blood levels. Epidemiology2009, 20, S253.
- Lin, Y.H.; Zhang, B.Y.; Lu, J.M.; Liu, J.; Guan, H.H.; Liu, Z.J.; Guo, S.W.; Zhao, S.M. Methylation of mercury and methylmercury metabolism in fish body. Fish. China1994, 18 (4), 326-329.
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; Aaseth, J.; Bjørklund, G. Sulfhydryl groups as targets of mercury toxicity. Chem. Rev2020, 417:213343.
- Jiang, Q.G.; Ji, Y.J.; Chang, Y.X. Environmental Chemical Poison Control Manual. Beijing: Chemical Industrial Press, 2004, pp. 1280.
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Rev. Toxicol1995, 25, 1-24.
- Clarkson, T.W.; Magos. L.; Myers. G.J. The toxicology of mercury--current exposures and clinical manifestations. Engl. J. Med2003, 349, 1731–1737.
- Carocci, A.; Rovito. N.; Sinicropi. M.S.; Genchi. G. Mercury toxicity and neurodegenerative effects. Environ. Contam. Toxicol2014, 229, 1-18.
- Ramon, R.; Murcia, M.; Ballester, F.; Rebagliato, M.; Lacasaña, M.; Vioque, J.; Llop, S.; Amurrio, A.; Aguinagalde, X.; Marco, A.; León, G.; Ibarluzea, J.; Ribas-Fitó, N. Prenatal exposure to mercury in a prospective mother-infant cohort study in a Mediterranean area, Valencia, Spain. Total Environ2008, 392, 69-78.
- Caserta, D.; Graziano, A.; Monte, G.L.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Rev. Med. Pharmaco2013, 17, 2198-2206.
- Gilbertson, M. Male cerebral palsy hospitalization as a potential indicator of neurological effects of methylmercury exposure in Great Lakes communities. Res2004, 95, 375–384.
- Chan, H.M.; Egeland, G.M. Fish consumption, mercury exposure, and heart disease. Rev2004, 62, 68-72.
- Stern, A.H. A review of the studies of the cardiovascular health effects of methylmercury with consideration of their suitability for risk assessment. Res2005, 98, 133–142.
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury exposure and heart diseases. J. Environ. Res. Public Health2017, 14, 74.
- Koos, B.J.; Longo, L.D. Mercury toxicity in the pregnant woman, fetus, and newborn infant. A review. Am. J. Obstet. Gynecol 1976, 126 (3), 390-409.
- Gunn, A.J.; Quaedackers, J.S.; Guan, J.; Heineman, E.; Bennet, L. The premature fetus: not as defenseless as we thought, but still paradoxically vulnerable? Neurosci2001, 23(3), 175-179.
- Stephen, J.G. To sea or not to sea: Benefits and risks of gestational fish consumption. Toxicol2008, 26, 81-85.
- Guan, M.; Zhang, L.; Zhang, G.R.; Le, J.; Guan, C.N.; Yue, H.Y.; Yu, G.Y. Determination and study of methylmercury content in five organs of fetuses at different gestational ages, Chinese J Public Health1997, 16, 87-89.
- Sakamoto, M.; Kubota, M.; Liu, X.J.; Murata, K.; Nakai, K.; Satoh, H. Maternal and fetal mercury and n-3 polyunsaturated fatty acids as a risk and benefit of fish consumption to fetus. Sci. Technol 2004, 38, 3860-3863.
- Zeilmaker, M.J.; Hoekstra, J.; van Eijkeren, J.C.H.; de Jong, N.; Hart, A.; Kennedy, M.; Owen, H.; Gunnlaugsdottir, H. Fish consumption during child bearing age: A quantitative risk-benefit analysis on neurodevelopment. Food Chem. Toxicol2013, 54, 30-34.
- Taylor, C.M.; Emmett, P.M.; Emond, A.M.; Golding, J. A review of guidance on fish consumption in pregnancy: Is it fit for purpose? Public Health Nutr2018, 21, 2149-2159.
- Gholami, N.; Abotorabi, S.; Lalooha, F.; Oveisi, S. Effects of fish oil supplementation on pregnancy outcomes in pregnant women referred to Kosar Hospital. J. Pharm. Res2020, 19 (3), 241-247.
- Dunstan, J.A.; Prescott, S.L. Does fish oil supplementation in pregnancy reduce the risk of allergic disease in infants? Opin. Allergy. CL2005, 5 (3), 215-221.
- Hansen, S.; Strøm, M.; Maslova, E.; Dahl, R.; Hoffmann, H.J.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Granström, C.; Halldorsson, T.I.; Chavarro, J.E.; Linneberg, A.; Olsen, S.F. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. Allergy Clin. Immunol2017, 139 (1), 104-111.
- Onwude, J.L.; Lilford, R.J.; Hjartardottir, H.; Staines, A.; Tuffnell, D. A randomised double blind placebo controlled trial of fish oil in high risk pregnancy. J. Obstet. Gynaecol1995, 102 (2), 95-100.
- Olafsdottir, A.S.; Skuladottir, G.V.; Thorsdottir, I.; Hauksson, A.; Thorgeirsdottir, H.; Steingrimsdottir, L. Relationship between high consumption of marine fatty acids in early pregnancy and hypertensive disorders in pregnancy. J. Obstet. Gynaecol2006, 113, 301-309.
- Zhou, S.J.; Yelland, L.; McPhee, A.J.;, Quinlivan, J.; Gibson, R.A.; Makrides, M. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. J. Clin. Nutr2012, 95 (6), 1378-1384.
- Wakita, Y. Hypertension induced by methyl mercury in rats. Appl. Pharmacol1987, 89, 144-147.
- Moreira, E.L.G.; Farina, M. An unsolved puzzle: the complex interplay between methylmercury and fish oil-derived fatty acids within the cardiovascular system. Res2014, 3 (5), 300-310.
- Merkle, S.; Giese, E.; Rohn, S.; Karl, H.; Lehmann, I.; Wohltmann, A.; Fritsch, J. Impact of fish species and processing technology on minor fish oil components. Food Control2017, 73, 1379-1387.
- Silbernagel, S.M.; Carpenter, D.O.; Gilbert, S.G.; Gochfeld, M.; Groth, E.; Hightower, J.M.; Schiavone, F.M. Recognizing and preventing overexposure to methylmercury from fish and seafood consumption: Information for physicians. Toxicol2011, 2011, 983072.
- Hsi, H.C.; Hsu, Y.W.; Chang, T.C.; Chien, L.C. Methylmercury concentration in fish and risk-benefit assessment of fish intake among pregnant versus infertile women in Taiwan. Plos One2016, 11 (5), e0155704.
- Razzaghi, H.; Tinker, S.C. Seafood consumption among pregnant and non-pregnant women of childbearing age in the United States, NHANES 1999–2006. Food Nutr. Res2019, 58, 23287.
- Schober, S.E.; Sinks, T.H.; Jones, R.L.; Bolger, P.M.; McDowell, M.; Osterloh, J.; Garrett, E.S.; Canady, R.A.; Dillon, C.F.; Sun, Y.; Joseph, C.B.; Mahaffey, K.R. Blood mercury levels in US children and women of childbearing age. 1999-2000. JAMA2003, 289, 1667-1674.
- World Health Organization (WHO). Children’s Exposure to Mercury Compounds. 2010. <https://www.who.int/publications/i/item/9789241500456>
- Food Standards Australia New Zealand (FSANZ). Mercury in Fish.viewed December 2020. <https://www.foodstandards.gov.au/consumer/chemicals/mercury/Pages/default.aspx>
- Shimshack, J.P.; Ward, M.B.; Beatty, T.K. Mercury advisories: Information, education, and fish consumption, Environ. Econ. Manage2007, 53, 158–179.
- Lando, A.M.; Zhang, Y.T. Awareness and knowledge of methylmercury in fish in the United States. Res2011, 111, 442–450.
- Oken, E.; Wright, R.O.; Kleinman, K.P.; Bellinger, D.; Amarasiriwardena, C.J.; Hu, H.; Rich-Edwards, J.W.; Gillman, M.W. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Health Perspect2005, 113, 1376–1380.
- Gale, C.R.; Robinson, S.M.; Godfrey, K.M.; Law, C.M.; Schlotz, W.; O’Callaghan, F.J. Oily fish intake during pregnancy—Association with lower hyperactivity but not with higher full-scale IQ in offspring. Child Psychol. Psychiatry2008, 49, 1061-1068.
- Amy, M.L.; Sara, B.F.; Conrad, J.C. Awareness of methylmercury in fish and fish consumption among pregnant and postpartum women and women of childbearing age in the United States. Res2012, 116, 85-92.
- Frithsen, I.; Goodnight, W. Awareness and implications of fish consumption advisories in a women's health setting. Reprod. Med2009, 54 (5), 267-272.
- Schmidt, L.; Bizzi, C.A.; Duarte, F.A.; Muller, E.I.; Krupp, E.; Feldmann, J.; Flores, E.M.M. Evaluation of Hg species after culinary treatments of fish. Food Control2015, 47, 413-419.
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; vom Saal, F.S. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Health Perspect2003, 111, 994–1006.
- US Food and Drug Administration (US FDA). Draft risk and benefit report: Section V Scientific basis for risk and benefit assessment. 2009. Available at: http://www.fda.gov/Food/FoodSafety/Product-SpecificInformation/Seafood/FoodbornePathogensContaminants/Methylmercury/ucm088758.htmS.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph192315929
