Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Food Science & Technology
|
Mycology
黑木耳多糖(AAP)因其独特的结构和生理活性而在医学和保健领域得到了广泛的研究。许多种类黑木耳多糖已通过不同的方法提取、分离和纯化,并对其结构进行了分析。黑木耳多糖已被证明对人体有益,包括减缓衰老过程、控制肠道系统和治疗心血管疾病。本文介绍了AAP的提取、分离和纯化黑木耳以及医学和医疗保健领域的研究,都指出了这些方法的缺点和局限性。我们还提出了未来的研究方向黑木耳多糖;必须确认标准化的加工方法,并且商业应用需要官方批准的AAP。最后,对AAP的发展给出了乐观的展望。
- Auricularia auricula
- polysaccharide
- extraction technology
1. 简介
耳蕈是食用菌,是中国的传统药物,也是世界第四大栽培蘑菇品种[1]。它因其营养和治疗特性而在亚洲和世界各地受到广泛重视。特别是中国是耳廓的主要生产国,耳廓菌已被食用至少4000年,在亚洲被用作常见的传统药物[2,3]。
耳廓富含多糖、蛋白质、脂肪、维生素、色素和微量元素[4]。耳廓多糖(AAP)是耳廓的主要活性成分,占总含量的60%[5,6]。近年来,医疗保健领域对AAPs的研究已经加强,并且发现许多慢性疾病对其治疗效果的反应相当有利[7]。
许多研究证实,耳廓多糖可以调节肠道菌群[8],增强免疫调节[9],减缓衰老过程[10,11],抵抗辐射[12],降低血脂[13],消灭病毒[14]。这些活动对营养药物和药物的发展显示出巨大的希望。AAP的健康益处远远超过耳廓中的其他必需营养素,是未来耳廓深加工的主要方向。
由于多糖是一种真菌杂多糖,其结构和组成的不确定性给其深入研究带来了很大的困难。不同提取或干燥方法获得的样品的生物活性不同[15]。因此,提取技术的研究是深入研究耳廓多糖的第一道坎。为了提高多糖的收率,近年来科学家开发了酶法提取[16]、超声提取[17]、液体发酵提取[18]等方法。就目前获得的多糖而言,主要研究了四种类型的多糖(角苣苔(ACP)、耳廓苔(AAP)、多耳苣苔(APP)和默氏多糖(MFP))[19]。寻找对提取样品结构和分子量具有高重现性的提取方法是AAP提取的重点研究方向。而且,上述提取耳廓多糖的传统方法导致大量的烹饪废液和大量的营养损失。最近的研究表明,剩余的耳廓残留物种中的黑色素具有巨大的资源开发潜力,能够在废物上重新培养其他植物物种,并促进从乳清蛋白分离物制备饮料[20,21,22]。耳苣苔残留物的再利用也将是未来的研究方向。
中国目前是世界上最大的耳廓生产国,占全球总产量的90%以上。2018年,耳苋(干货)产量达到67.4万吨,产值374.6亿元,创汇61.5亿元。作为我国特色优势农产品,蘑菇在提高农业效益、增加农民收入、助力行业扶贫等方面发挥着重要作用[20]。需要注意的是,耳廓栽培的生产规模逐年增加,随着套袋栽培技术的普及应用,耳木林产品的市场压力也在不断增加[21]。耳廓种植规模不断扩大,产量逐年增加,市场销售利润空间逐渐萎缩。从卖方市场到买方市场正在逐步转变。耳廓深加工是耳廓产品未来发展方向之一。图1显示了2016年至2022年中国耳廓产量的变化,自2018年以来一直在稳步增长,到2020年达到7,295,900吨,同比增长3.96%[22]。到2022年,中国的耳廓产量将增长到7,723,400吨,同比增长1.98%[23]。随着栽培技术和培养方法的不断更新,耳廓多糖产品的进一步发展及其深加工是耳木耳产业的前进方向。如图2所示,在所有耳廓深加工产品中,耳廓饮料产品的市场份额最大,为28%,远高于其他产品。市场份额第二高的产品是耳廓营养粉,市场份额为8%,其次是耳廓粥和即食耳廓,各占6%的市场份额[22,24,25]。因此,在中国市场,耳廓主要用于有助于降低血脂和增强免疫力的产品中。
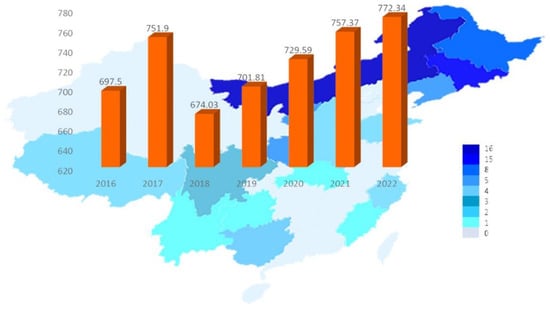
图1.2016-2022年中国耳廓菌产量与预测.
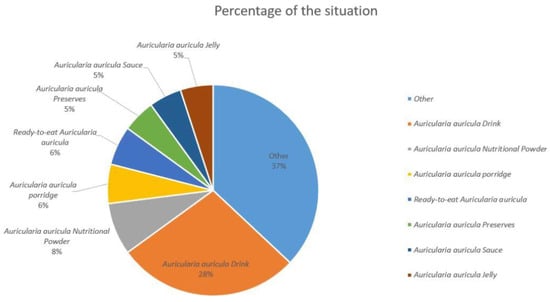
图2.耳廓市场的商品类型比例。
作为耳廓多糖的营养和功能成分的需求正在增长,市场正在扩大[26,27]。许多高价值(AAP)衍生物已经开发出来,加工技术已经成熟。虽然它们对健康有很多好处,但它们还没有完全上市。耳廓多糖具有独特的结构,具有广泛的生物学和药理活性。由于其高含量、易于提取、特定结构、轻微的副作用和显着的治疗潜力,它们在世界范围内越来越受欢迎。到目前为止,已经从分布在世界各地的不同黑曲霉物种中提取了许多AAP[11]。对不同黑曲霉物种的AAP的结构和生物学和药理活性进行了大量深入研究。然而,标准化的加工方法尚未得到确认,AAP的商业应用需要官方批准。
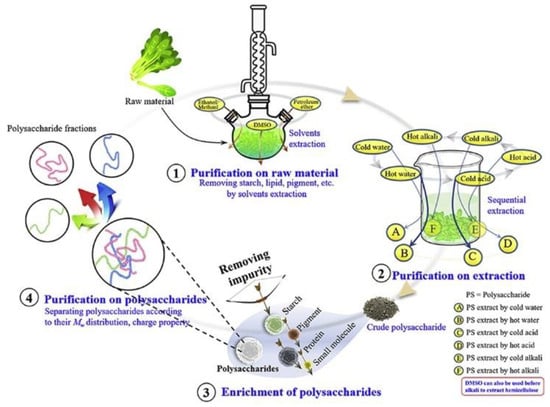
2. 分离纯化
多糖纯化的意义在于去除小分子,如色素和无机盐。目前,分离方法包括色谱和透析。纯化方法可以根据分子量和中等酸度的标准对多糖进行分类。由于其生物活性和无毒或低毒特性,食品来源的天然多糖在食品、化妆品、生物材料等领域越来越受欢迎[37,38,39,40,41,42]。纯化还可以进行可用、安全和可重复的多糖研究[43]。纯化方法的选择取决于多糖的性质,并受制造工艺的影响。在原料预处理过程中未完全去除的杂质(淀粉、脂质和色素)可能在随后的多糖提取过程中与多糖混合。此外,最近的研究表明,不同的提取条件,如pH和溶剂温度,都会导致不同多糖的渗透[44,45]。如图4所示,Wei等人[40]将多糖的分离分为三个阶段:原料中的多糖,粗提物中的多糖和粗多糖。粗多糖的纯化包括多糖的富集和具有不同结构或构象特征的馏分的分离。

Figure 4. The purification process of polysaccharides.
Polysaccharides have been widely accepted as nutraceuticals, which improve the immune function of the body. However, many polysaccharides remain as health products and fail to be developed into drugs. The main reason for this is that the separation and purification of polysaccharides is difficult, and the current technology level does not meet the requirements for this. Generally speaking, polysaccharides are hydrophilic macromolecules. The separation and purification methods of polysaccharides are different from those of small molecules. In addition, different polysaccharides have different properties, so different separation and purification methods must be used. This work requires not only a theoretical knowledge of polysaccharides, but also accumulated working experience in the separation and purification of polysaccharides.
2.1. Concentration Grading Method
This method mainly takes advantage of the fact that the solubility of different polysaccharides in different concentrations of organic solvents is different. The solubility of polysaccharides with larger molecular weights in ethanol or acetone are less than it is of those with a smaller molecular weight [46]. Therefore, the molecular weight of the product can be controlled by adjusting the concentration of the organic solvent. This is usually done in the following manner:
While stirring, a high concentration of anhydrous ethanol is slowly added to the solution of the polysaccharide mixture to reach a final concentration of 25% ethanol (v/v). After the addition of ethanol, the solution is left for 2 h and then centrifuged to obtain the supernatant and precipitate (which may be referred to as the “first precipitate”). The precipitate is of a high MW polysaccharide grade. While stirring, ethanol is slowly added to the supernatant to reach a final concentration of 35% ethanol (v/v). The solution is left for 2 h and then centrifuged to obtain the supernatant and precipitate (which may be referred to as the “second precipitate”). The second precipitate is also a polysaccharide fraction, but its MW is lower than the first precipitate. The step-down process can be carried out further, depending on circumstances. The key to graded precipitation is to avoid co-precipitation as much as possible. The concentration of the polysaccharide mixture should not be too high, the ethanol should not be added too fast, and the pH of the solution should be near neutral. The lower the concentration of polysaccharide solution, the weaker the co-precipitation effect and the better the purification effect. However, if the polysaccharide concentration is too low, the recovery of the polysaccharide will be reduced and the consumption of ethanol will be greatly increased. Usually, the concentration of polysaccharide in the mixture is adjusted from 0.25% (w/v) to 3% (w/v) before using this method [47,48,49,50]. The concentration grading method is commonly used in the research and development of polysaccharide nutraceuticals because it is much easier than column chromatography.
2.2. Column Chromatography Method
Column chromatography is the most widely used method for the purification of polysaccharides. Several methods of column chromatography are described, as follows:
2.2.1. Macroporous Resin
Macroporous adsorption resin is used to selectively adsorb organic substances from the solution by physical adsorption so as to achieve separation and purification. Its physical and chemical properties are stable; it is insoluble in acids, bases, and organic solvents, has good selectivity for organic substances, and it is unaffected by the presence of inorganic salts and strong ions, low molecular compounds, and swelling in water and organic solvents by the adsorption of solvents. The adsorption of macroporous resin relies on the van der Waals gravitational force between it and the adsorbed molecules (adsorbent), and works through its huge specific surface for physical adsorption, so that organic compounds can be separated by a certain solvent elution according to the adsorption force and its molecular weight size to achieve different purposes such as separation, purification, de-hybridization, and concentration. Macroporous resin can remove proteins, flavonoids, and pigments from polysaccharide solutions [51,52,53,54].
2.2.2. Cellulose Column Chromatography
Cellulose is a common filling material in columns. First, after waiting for swelling, activation is performed using 0.5 mol/L NaOH with 0.5 mol/L HCL solution; the cellulose in the column is equilibrated with NaCL solution, and then the polysaccharide is loaded onto the cellulose column for purification. Afterwards, the cellulose columns are eluted separately using an eluent so that different polysaccharide levels can be continuously eluted [46]. Polysaccharides can be separated according to different molecular weights or acid-based groups. In the elution process, the various polysaccharide fractions undergo several dissolution and precipitation processes in the cellulose column, and can eventually be separated from each other. This method can be called the “graded dissolution method”, which is basically the opposite of the graded precipitation method. Due to the high number of theoretical plates in the cellulose column chromatography, the purity of the eluate is higher [55]. However, the disadvantage of this method is the low flow rate and the long period of time required. The flow rate seems to be too low, especially for highly viscous acidic polysaccharides.
2.2.3. Gel Column Chromatography
Gel column chromatography is based on the size and shape of polysaccharide molecules, i.e., the molecular sieve principle, to separate polysaccharides. This chromatographic method is widely used for the separation and purification of polysaccharides. In general, the crude polysaccharides obtained are first purified using macroporous resin and cellulose chromatography, and are then further purified using gel column chromatography. Commonly used gels are various types of Sephadex, Sepharose, Bio gels, and later Sephacryl, Superdex, and Superose. The eluents are salt solutions and buffers of various concentrations.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28020582
This entry is offline, you can click here to edit this entry!
