Oncolytic virotherapy (OVT) has received significant attention in recent years, especially since the approval of talimogene Laherparepvec (T-VEC) in 2015 by the Food and Drug administration (FDA). Mechanistic studies of oncolytic viruses (OVs) have revealed that most, if not all, OVs induce direct oncolysis and stimulate innate and adaptive anti-tumour immunity. With the advancement of tumour modelling, allowing characterisation of the effects of tumour microenvironment (TME) components and identification of the cellular mechanisms required for cell death (both direct oncolysis and anti-tumour immune responses), it is clear that a “one size fits all” approach is not applicable to all OVs, or indeed the same OV across different tumour types and disease locations. This article will provide an unbiased review of oncolytic reovirus (clinically formulated as pelareorep), including the molecular and cellular requirements for reovirus oncolysis and anti-tumour immunity, reports of pre-clinical efficacy and its overall clinical trajectory. Moreover, as it is now abundantly clear that the true potential of all OVs, including reovirus, will only be reached upon the development of synergistic combination strategies, reovirus combination therapeutics will be discussed, including the limitations and challenges that remain to harness the full potential of this promising therapeutic agent.
- reovirus
- oncolytic virus
- pelareorep
- oncolytic virotherapy
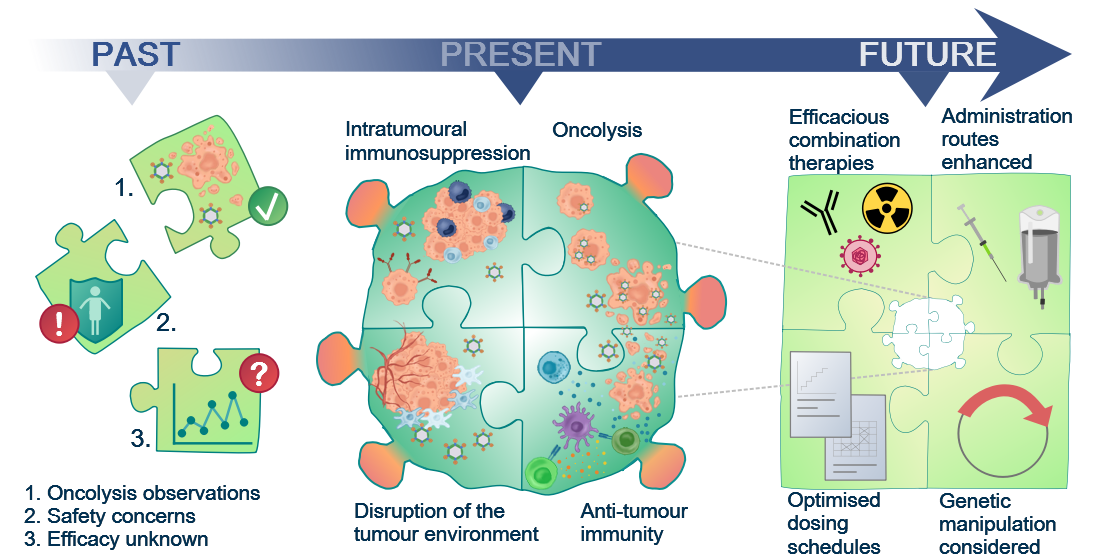
Figure 1. Although the oncolytic capacity of various viruses was noted already in the 1950s, development of oncolytic viruses (OVs) as therapeutic agents was limited by safety and efficacy concerns for a long time. With a greater understanding of virology and molecular techniques, the interest in OVs has peaked in recent years and a number of viruses, including reovirus, are now in clinical development. Today, we know that OVs exert their anti-tumour effects in several ways, e.g. direct oncolytic killing through replication in malignantly transformed cells; innate and adaptive anti-tumour immunity; disruption of the local tumour microenvironment sustaining the tumour; and intratumoural immunosuppressive effects, e.g. recruitment of regulatory T cells and enhanced expression of immune checkpoints on tumour cells. This knowledge will allow future initiatives to the identify the most efficacious combination therapies, optimised dosing schedules, and administration routes required for the success of reovirus as a therapeutic agent.
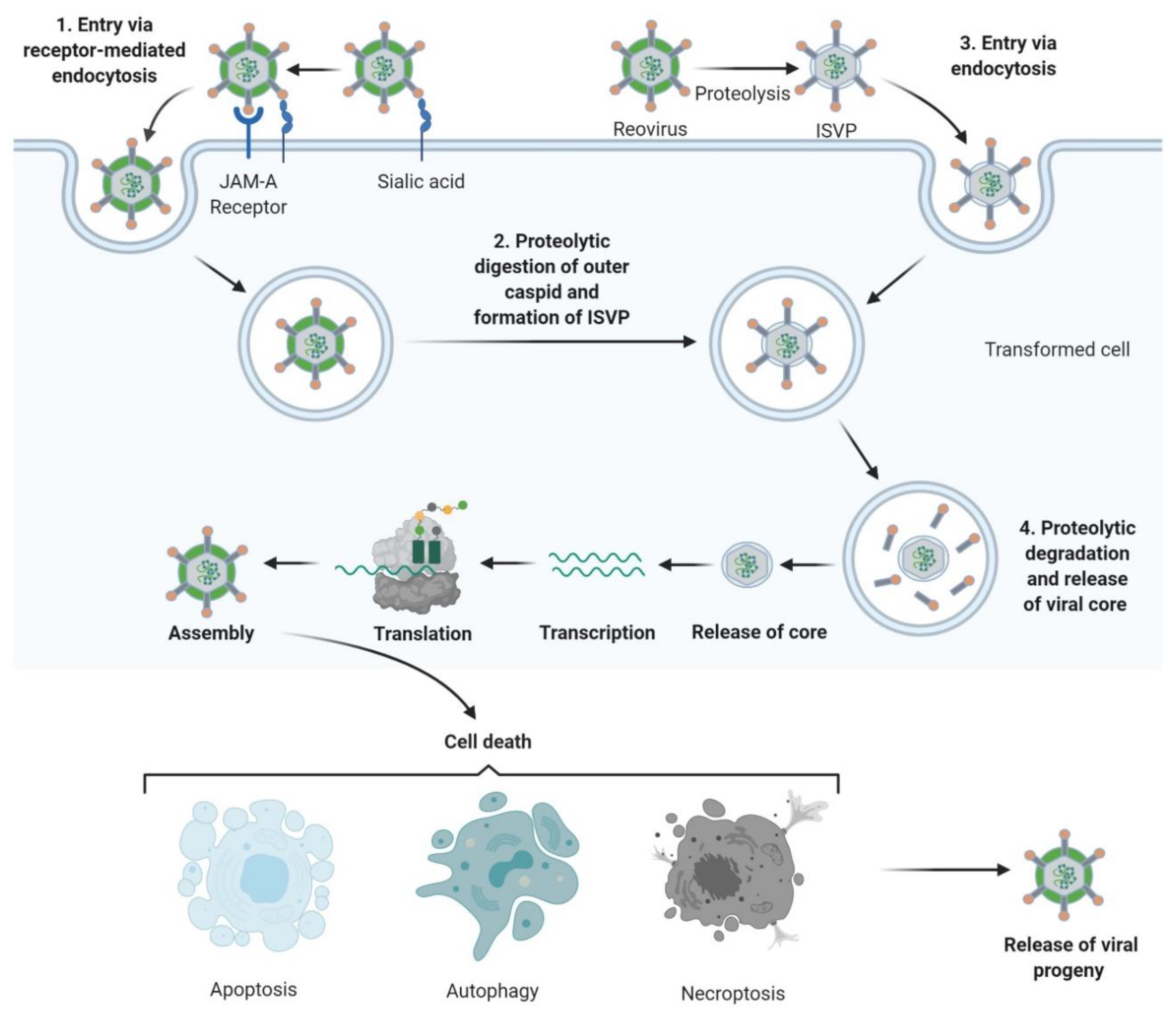
Figure 2. Reovirus replication: 1. Reovirus is first tethered via a weak interaction between σ1 and cell surface sialic acid; σ1 then binds with high affinity to junctional adhesion molecule A (JAM-A) resulting in internalization of the virus via receptor-mediated endocytosis. 2. Once internalized, the virus is transported to early and late endosomes where it undergoes proteolytic digestion to remove the outer capsid protein σ3 resulting in the formation of infectious subvirion particles (ISVPs). 3. Alternatively, ISVPs may be formed by extracellular proteases within the tumour environment allowing direct entry into cells via membrane penetration. 4. After further proteolytic degradation a transcriptionally active viral core is released into the cytoplasm. Transcription and translation occur ultimately leading to the assembly of new viral progeny, host cell death and progeny release. Figure created using Biorender (https://biorender.com/).
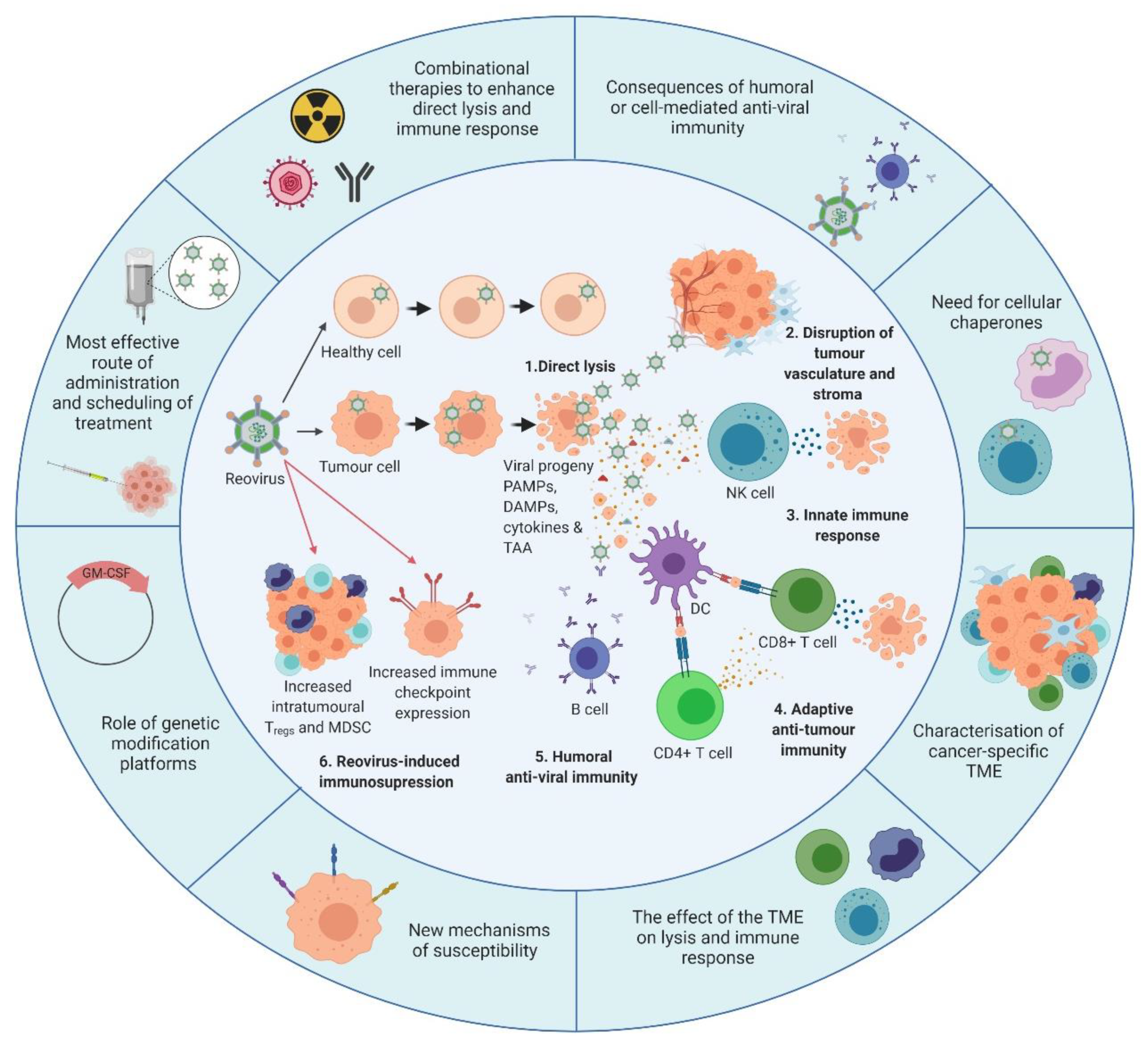
Figure 3. Overview of reovirus mechanisms of action and the developments required. The inner circle illustrates what is currently known about reovirus. 1. In healthy cells, anti-viral immune responses limit reovirus replication and prevent lytic killing. By contrast, oncogenic signalling pathways render tumour cells susceptible to reovirus replication and direct oncolysis. 2. Reovirus replicates in the tumour vasculature and stroma due to reciprocal cell:cell interactions which alter anti-viral signalling. 3. Infection of tumour cells leads to the release of viral progeny, cytokines and tumour-associated antigens (TAAs), which initiates innate anti-tumour immunity including cytokine-mediated bystander killing and natural killer (NK) cell-mediated cytotoxicity. 4. Adaptive anti-tumour immunity is generated following the phagocytosis of TAAs by dendritic cells (DCs) and presentation of TAAs to CD4+ve and CD8+ve T cells, which facilitates priming of tumour-specific cytotoxic T lymphocytes (CTLs). 5. In addition to innate and adaptive anti-tumour immune responses, humoral anti-viral immunity is induced, leading to the production of reovirus-specific neutralising antibodies (NAbs). 6. Following induction of anti-tumour/anti-viral immune responses, regulatory immune mechanisms are “switched-on” to control ongoing immune responses, including upregulation of immune checkpoints and increased levels of regulatory T cells (Tregs) and/or myeloid-derived suppressor cells (MDSCs). The outer circle highlights priority research areas to improve reovirus efficacy. These include gaining a greater understanding of: (i) the consequence of humoral and/or cell-mediated anti-viral immunity on reovirus efficacy which would inform the development of, or the requirement for, cellular chaperones; (ii) the tumour microenvironment (TME) and how it influences reovirus oncolysis and anti-tumour immunity; (iii) the cellular determinants utilized by reovirus for direct oncolysis, including mechanisms of reovirus resistance; (iv) the potential benefits of genetically-modified reovirus platforms; (v) reovirus scheduling to maximize virus delivery and efficacy including the best route of virus administration; and (vi) combinatorial approaches that are designed to boost both direct oncolysis and anti-tumour immune responses. PAMPs: pathogen-associated molecular patterns; DAMPs: damage-associated molecular patterns; GM-CSF: granulocyte-macrophage colony stimulating factor. Figure created using Biorender.com.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12113219
