Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Allergy
Cancer has become one of the deadliest diseases in our society. Surgery accompanied by subsequent chemotherapy is the treatment most used to prolong or save the patient’s life. Still, it carries secondary risks such as infections and thrombosis and causes cytotoxic effects in healthy tissues. Using nanocarriers such as smart polymer micelles is a promising alternative to avoid or minimize these problems.
- smart polymeric micelles
- anticancer hydrophobic drugs
- nanocarriers
1. Introduction
Cancer is one of the leading causes of death worldwide. Surgery accompanied by subsequent chemotherapy is the most widely used treatment to lengthen or save the patient’s life. Surgery inherently bears secondary risks, such as infection and thrombosis. Chemotherapeutic compounds may be ineffective due to low water solubility, low tumor targeting, and low cellular uptake, and they may cause cytotoxic effects on healthy tissues [1]. In cancer treatment, nanocarrier systems have great potential to help or replace traditional chemotherapy. Controlled drug delivery by nanoparticles is crucial but has always been a significant challenge in developing new effective cancer therapies and diagnostics. The nanocarriers most used for these purposes are carbon nanotubes [2], dendrimer [3], micelles [4], quantum dot [5], liposomes [6], cubosomes [7], magnetic nanoparticles [8], gold nanoparticles, and mesoporous silica nanoparticles (Figure 1) [9,10].
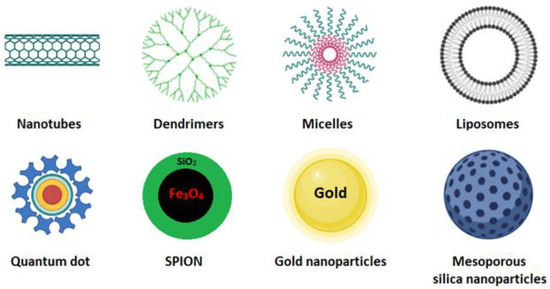
Figure 1. Schematic representation of some nanocarriers currently used as smart drug-delivery systems [10].
These systems, also known as smart drug-delivery systems (SDDS), are developed so that affected cells, but not healthy cells, locally deliver therapeutic agents at the right concentration. The release of these agents occurs by physicochemical mechanisms that depend on external or internal stimuli that act on the nanoparticles [11,12]. These stimuli include pH gradients [13], redox reactions [14], enzymatic cleaving [15], temperature [16,17,18], ultrasound waves [19], light [20], and magnetic and electric fields [8,21].
All these nanocarriers, in one way or another, always contain a hydrophilic polymer to (i) improve their solubility or stability in extracellular fluids, (ii) avoid processes of exclusion by the immune system and rapid elimination by the reticuloendothelial system, and (iii) functionalize molecules (peptides, proteins, antibodies, genetic material, etc.) that allow the drug to be guided to the target and to be released. This conjugation is always done by forming amide, disulfide, or ester bonds [10]. Within SDSS, polymeric micelles have gained renewed interest due to their physicochemical properties that optimize accumulation in tissues caused by the increased vascular permeability in sites of cancer or inflammation, the so-called enhanced permeation, and the retention (EPR) effect that regulates tumor targeting. Furthermore, polymeric micelles notably increase the solubility of hydrophobic drugs in water by 10- to 5000-fold. The drugs are stabilized in a core composed of a hydrophobic polymeric or lipid matrix covered by a hydrophilic polymer. This modification will prolong the circulation of drugs in the bloodstream. The synthesis of micelles as an SDDS constitutes a potential research topic because of the wide variety of biocompatible and biodegradable polymers and lipids [22,23].
2. Polymeric Micelles
Polymeric micelles are formulated by self-assembly in an aqueous medium of block copolymers that spontaneously form a core–shell structure with a marked amphiphilic character. The differences between the hydrophobic and hydrophilic segments will directly influence the micellization process for forming this type of nanostructures. Copolymers are classified according to the number of monomers in their structure, such as di-, tri-, tetra-, and multiblock copolymers.
Polymeric micelles generally have a hydrophobic core with a hydrophilic coating. Most therapeutic cancer agents are hydrophobic and can therefore be encapsulated in the core. These copolymers can be modified unlimitedly, making them either more hydrophobic or hydrophilic, depending on the chemical properties of the drug under study. In this way, the stability and solubility of these drugs in biological systems can be improved [24]. Figure 2 shows the most frequently used polymers as a hydrophobic core.
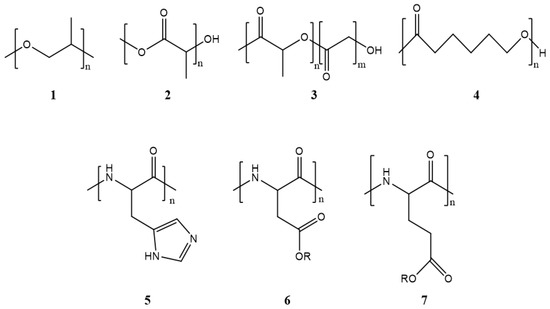
Figure 2. Structure of the most frequently used polymers as a core in the micelle’s preparation. Polyethers; 1: poly(propylene oxide) (PPO) [25]. Polyesters; 2: poly(L-lactide) (PLA) [26], 3: poly(lactide-co-glycolide) (PLGA), 4: poly(ε-caprolactone) (PCL) [27], 5: poly(L-histidine)(pHis) [28], 6: poly(L-aspartic acid) and derivatives (pAsp) [29], 7: poly(L-glutamic acid) and derivatives (pGlu) [30].
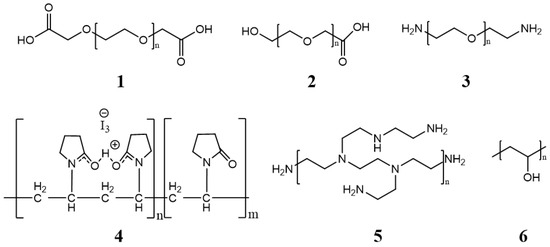
The core is covered by a layer generally composed of low toxic hydrophilic polymers, stabilizing the system in an aqueous medium. The properties of the cover layer determine how efficiently micelles can circulate in the bloodstream. Figure 3 shows some of the most commonly used polymers [31].
The formation of micelles depends on three parameters: ionic strength, critical micellar temperature (CMT), and critical micellar concentration (CMC). Ionic strength is relevant in most cases when working with ionic surfactants (whose polar group has a charge). The critical micellar temperature (CMT) is nothing more than that temperature above which the formation of micelles is favored. The critical micellar concentration (CMC) is defined as the concentration of surfactants above which micelles are formed.
The formation of the micelles will depend on attractive forces that lead to the association of molecules and repulsive forces that control the growth of the nanostructure. CMC depends on the block copolymers’ hydrophobic–hydrophilic balance, the polymers’ molecular weight, and their chemical properties [36]. Micelle formation occurs by removing hydrophobic parts from the aqueous medium and re-establishing hydrogen bonds in water (Figure 4).
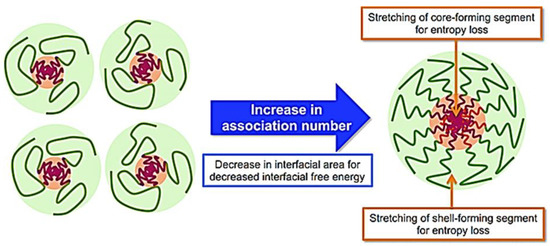
Figure 4. Mechanism of micelle formation from copolymers [37].
These bonds cause a decrease in the interfacial free energy of the system and a greater stabilization of the core. The micellization process is expressed in terms of free energy, CMT, and CMC (Equation (1)) as:
where ΔG0 is the standard free-energy change of micellization, R is the gas constant, and T is the absolute temperature of the system [38].
ΔG0 = RT ln(CMC)
Using amphiphilic copolymers with a low CMC value ensures that polymeric micelles become more stable in blood than liposomes and other surface-active micelles. In some cases, the formation of polymeric micelles is temperature dependent. The temperature of the formulation must always be higher than the critical micellar temperature (CMT), which depends on the structures and properties of the copolymers [39]. The number of surfactant monomers that form a micelle is defined as the micellar association number, which is thermodynamically defined and varies from 10 to 200 monomers [40].
3. Micelle Synthesis
Five techniques are used to synthesize micelles. These are (1) direct solution, (2) oil in water emulsification, (3) thin-film hydration/solvent evaporation (4) dialysis, and (5) freeze-drying (Table 1). The applied technique depends on the solubility of the selected copolymer. The polymeric micelle is classified as regular or direct if the copolymer’s hydrophilic part is exposed on its surface. On the other hand, the micelles are called inverse when they do not have a copolymer with the hydrophilic part exposed on the surface [10].
Table 1. Preparation methods of the polymeric micelles [31].
| Methods | Advantage/Disadvantage | Drug-Loading Capacity | Solvents | Types of Drugs | Encapsulated Anticancer Drug | Polymers Used |
|---|---|---|---|---|---|---|
| Direct dissolution | The simplest technique to prepare polymeric micelles. Does not use organic solvents. Low-molecular-weight hydrophilic polymers | Low drug-loading capacity due to water solubility of polymers | Water | Not applicable for most hydrophobic drugs | Paclitaxel [41] | Mostly hydrophilic polymers; PLA-PEG |
| Docetaxel [42] | d-a-tocopheryl PEG1000 succinate (TPGS) | |||||
| Doxorubicin [43] | Pluronic F127/poly (methyl vinyl ether-alt-maleic acid) | |||||
| Oil-in-water emulsification | Easy preparation. Small particles with a narrow size distribution. Not environmentally friendly due to the use of chlorinated organic solvents. | High drug-loading capacity | Organic solvents immiscible in water (CHCl3, EtAc, and CH2Cl2) | Hydrophobic drugs | Doxorubicin and erlotinib [44] | PLGA/pluronic F-127 |
| Triptorelin [45] | PLA/PLGA | |||||
| Thin-film hydration/solvent evaporation | Only applicable for copolymers with high hydrophilic–lipophilic balance (HLB). Feasible for scaling up but very expensive | High drug-loading capacity and encapsulation efficiency | Water-miscible volatile organic solvents (DMF, THF, DMSO, acetonitrile, MeOH, acetone) | Hydrophobic drugs | Doxorubicin [46] | PEG 5000-lysine-di-tocopherol succinate (P5kSSLV) |
| Curcumin [47] | Poly(ethyleneoxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-b-PPO-b-PEO/pluronic F-127) | |||||
| Paclitaxel [48] | Inutec SP11 (INT) | |||||
| Dialysis | For highly hydrophobic polymers with long alkyl chains. Difficulty releasing. Easy to remove organic solvents. Not applicable on a large scale due to high water consumption. | High drug-loading capacity | Water-miscible volatile organic solvents (DMF, THF, DMSO, acetonitrile, MeOH, acetone) | Hydrophobic drugs | Docetaxel [49] | PEG/hyperbranched poly(amidoamine) HAPH |
| Docetaxel [50] | PLGA/PEG–maleimide | |||||
| Doxorubicin [51] | PCL-S-S- biodegradable photoluminescent polymer (BPLP) | |||||
| Freeze-drying | High stability and narrow size distribution. Organic-solvent reusability. Thermolabile drug-encapsulation suitability. Limited lyophilize organic solvents and copolymers soluble in them. | High drug-loading capacity | The mixture of water and freeze-dryable organic solvents such as tert-butanol and dimethyl acetamide | Hydrophobic drugs | TM-2 [52] | mPEG/PLA |
| Docetaxel [53] | Thermosensitive methoxy poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl) methacrylamide lactate] (mPEG-bpHPMAmLacn) |
4. Size
The classification of micelles by their size has been the subject of debate. Some authors, such as Qi et al., support the argument that micelles smaller than 30–50 nm are classified as small, simple micelles. Micelles larger than 50 nm are classified as large micellar complexes. As in other nanostructured systems, small micelles generally experiment with a single-step aggregation process during their formation. However, the aggregation process is usually accompanied by several steps in the case of large and complex micelles [54]. The size of a specific micelle depends on the compacting of the copolymer chains. The lengths, the relationship between the hydrophobic and hydrophilic blocks, and the molecular weight of the surfactants will determine the micellar association number and, therefore, the total size of the micelle.
Crothers et al. demonstrated experimentally how the values of the mass-average association numbers of the micelles (Nw) determine the shape of the micelle nucleus and its size. If Nw reaches high values, micelles tend to form a rod or worm shape [55]. The size of the charged micelles or ionic micelles can be modified by adding salts such as potassium bromide and sodium salicylate. These salts can neutralize the charges on the micellar surface, promoting a pronounced growth of the micelles [56]. The particle size has an important effect on the immune system. Small micelles have high blood circulation and do not cause many activations of the immune system. If their size is less than 20 nm, the renal system filters them rapidly out of circulation before they reach their goal. Generally, it is recommended that the hydrodynamic diameter be between 60 to 150 nm to obtain effective drug delivery and release [57].
5. Surface Charge
Most micelles used in drug delivery are in the colloidal state and will therefore have a surface charge (positive, negative, or neutral). The surface charge of these nanocarriers can be changed by chemically modifying their surface with hydrophilic polymers, amino acids, or peptides. The dynamic light-scattering technique is used to determine the surface charge of micelles by Z-potential measurements. The higher the value of the modular Z-potential, the better the particles’ colloidal stability and, thus, a higher useful life [58]. Surface charge is undoubtedly important in determining protein adsorption and cellular interactions. In vitro studies show improved cellular uptake and circulation time for charged micelles. Neutral micelles have a longer circulation time but also greatly inhibit plasma proteins’ adsorption to the particle surface. Positively charged micelles have better interaction with cell membranes and internalization but could sometimes have a toxic effect on cells. The negative charge has reflected milder toxicity but decreases the cellular absorption of particles [59]. Positively charged nanostructured systems (<150 nm) tend to accumulate in the liver. However, if these systems are negatively charged, their circulation will increase and their accumulation will be more difficult. Particles greater than 150 nm are retained in the spleen [35].
Researchers must reach a consensus between cellular absorption, circulation times, and toxicity of micelles when their surface charge is modified. Kalinova et al. prepared new curcumin-loaded micelles with tunable surface charges. Copolymers were designed using poly(D,L-lactide) and poly((2-dimethylamino)ethyl methacrylate) and poly(oligo(ethylene glycol)methyl ether methacrylate). The charges varied from strongly positive to neutral as the concentration of hydrophilic polymer was increased. The release profiles show that the curcumin was released much faster for the neutral micelles, but these had lower colloidal stability than the positively charged micelles [60]. Xiao et al. prepared micelles loaded with paclitaxel through the self-assembly of new copolymers called telodendrimers, which are formed by polyethylene glycol (PEG) and cholic dendritic acids (CA). The surface-charge effects were studied by conjugating anionic D-aspartic acids (D) or cationic D-lysines (K) at the ends of the PEG chain. Macrophages do not specifically take up micelles with a high negative and positive surface charge. However, micelles with less negative surface charge showed preferential uptake at the tumor site. The slightly negative charge was also able to prevent the rapid removal of micelles from the bloodstream [61].
6. Shape
The morphology of block copolymer micelles in dilute solution is generally spherical. The lower-energy spherical conformation can minimize the hydrophobic head’s exposure to the bulk aqueous phase. Different morphologies can be obtained by a remarkable increase in the concentration of surfactants, or the reaction temperature increases excessively. Sphere, rod, and star micelles have been accepted as a function of the length of the hydrophobic/hydrophilic blocks, the concentration of surfactants, and the solvent used.
Kimura et al. prepared different micelles with spherical and rod morphology by varying chain length and degree of polymerization (DP). Homopolymers with a lower degree of DP formed spherical micelles through the multi-chain assembly, whereas homopolymers that were longer than a threshold DP formed rod micelles [37]. Star-shaped micelles have gained particular interest because the polymeric arms provide a structure with excellent solubility and functionality to guide the drug into a tumor environment [39]. Kang et al. created a new pH-sensitive micellar system that reversibly changes its morphology from spherical to worms. The authors reacted cetyltrimethylammonium bromide (CTAB), 4-hydroxybenzaldehyde (HB), and p-toluidine (MB) by a dynamic covalent-bonding hydrotropic mechanism. If the pH increased, the viscosity of the solution first decreased and then increased rapidly. The microscopic micellar aggregates changed from a spherical morphology to a worm morphology. This morphological change is reversible because when the pH of the medium decreases, the particles become spherical again [62].
Li Deng et al. synthesized an amphiphilic copolymer based on poly (ε-caprolactone) (PCL) and hydrophilic P (2-(2-methoxyethoxy) ethyl methacrylate and oligo (ethylene glycol) methacrylate (MEO2MA-co-OEGMA)). Micelles were thermo-magnetically sensitive, presenting a lower critical solution temperature (LCST) around 43.5 °C with potential applications in magnetic hyperthermia and the release of drugs facilitated by magnetothermia [63]. Shang et al. prepared another system with a four-armed star morphology. Each star tip was covered by a folic-acid (FA) molecule, which gave the micellar structure greater internalization and more effective active targeting [64].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15010004
This entry is offline, you can click here to edit this entry!
