Nipah virus (NiV) infection is a viral disease caused by a Henipavirus, belonging to the Paramyxoviridae family, responsible for a zoonosis. The course of the disease can be very serious and lead to death. NiV natural hosts are fruit bats (also known as megabats) belonging to the Pteropodidae family, especially those of the Pteropus genus. Natural infection in domestic animals has been described in farming pigs, horses, domestic and feral dogs and cats. Natural NiV transmission is possible intra-species (pig-to-pig, human-to-human) and inter-species (flying bat-to-human, pig-to-human, horse-to-human).
- Nipah virus
- pathology
- vaccines
- epidemiology
- diagnostics
- legislation
- pathogenesis
- immune response
- geographic information system
- spillover events
1. Introduction
2. Biological Features of the Nipah Virus (NiV)
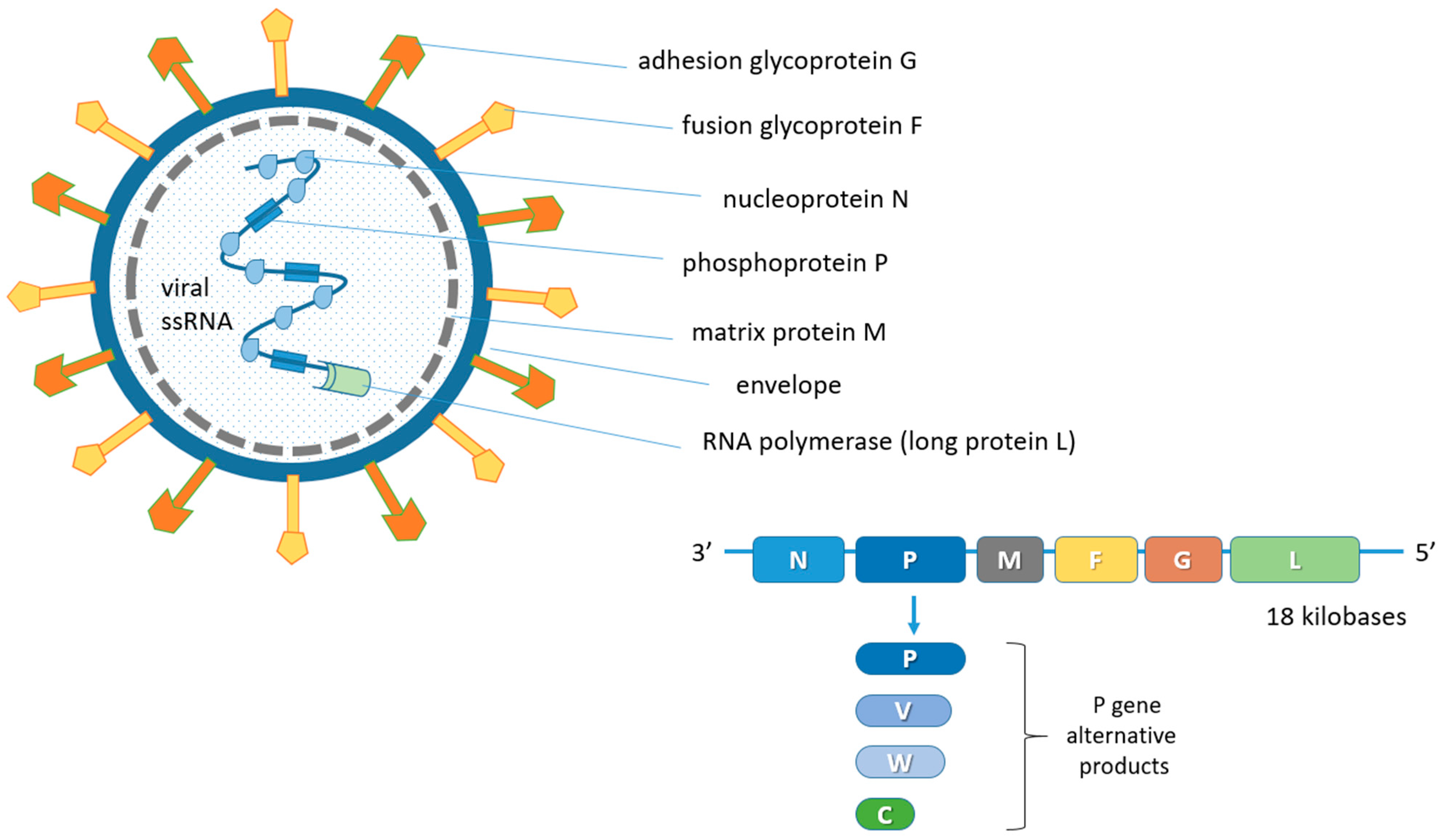
3. Host Range
4. Climate and Anthropogenic Influence on Pteropus Bats and NiV Spillover Events
This entry is adapted from the peer-reviewed paper 10.3390/ani13010159
References
- Yuen, K.Y.; Fraser, N.S.; Henning, J.; Halpin, K.; Gibson, J.S.; Betzien, L.; Stewart, A.J. Hendra virus: Epidemiology dynamics in relation to climate change, diagnostic tests and control measures. One Health 2021, 12, 100207.
- Mohd Nor, M.N.; Gan, C.H.; Ong, B.L. Nipah virus infection of pigs in peninsular Malaysia. Rev. Sci. Tech. 2000, 19, 160–165.
- Aditi; Shariff, M. Nipah virus infection: A review. Epidemiol. Infect. 2019, 147, e95.
- Thanapongtharm, W.; Linard, C.; Wiriyarat, W.; Chinsorn, P.; Kanchanasaka, B.; Xiao, X.; Biradar, C.; Wallace, R.G.; Gilbert, M. Spatial characterization of colonies of the flying fox bat, a carrier of Nipah virus in Thailand. BMC Vet. Res. 2015, 11, 81.
- Singh, R.K.; Dhama, K.; Chakraborty, S.; Tiwari, R.; Natesan, S.; Khandia, R.; Munjal, A.; Vora, K.S.; Latheef, S.K.; Karthik, K.; et al. Nipah virus: Epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies—A comprehensive review. Vet. Q. 2019, 39, 26–55.
- Pillai, V.S.; Krishna, G.; Veettil, M.V. Nipah virus: Past outbreaks and future containment. Viruses 2020, 12, 465.
- Satterfield, B.A.; Dawes, B.E.; Milligan, G.N. Status of vaccine research and development of vaccines for Nipah virus. Vaccine 2016, 34, 2971–2975.
- Devnath, P.; Wajed, S.; Chandra Das, R.; Kar, S.; Islam, I.; Masud, H.M.A.A. The pathogenesis of Nipah virus: A review. Microb. Pathog. 2022, 170, 105693.
- Pedrera, M.; Macchi, F.; McLean, R.K.; Franceschi, V.; Thakur, N.; Russo, L.; Medfai, L.; Todd, S.; Tchilian, E.Z.; Audonnet, J.C.; et al. Bovine herpesvirus-4-vectored delivery of Nipah virus glycoproteins enhances T cell immunogenicity in pigs. Vaccines 2020, 8, 115.
- Mungall, B.A.; Middleton, D.; Crameri, G.; Bingham, J.; Halpin, K.; Russell, G.; Green, D.; McEachern, J.; Pritchard, L.I.; Eaton, B.T.; et al. Feline model of acute Nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J. Virol. 2006, 80, 12293–12302.
- Pallister, J.A.; Klein, R.; Arkinstall, R.; Haining, J.; Long, F.; White, J.R.; Payne, J.; Feng, Y.R.; Wang, L.F.; Broder, C.C.; et al. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol. J. 2013, 10, 237.
- Weingartl, H.M.; Berhane, Y.; Caswell, J.L.; Loosmore, S.; Audonnet, J.C.; Roth, J.A.; Czub, M. Recombinant Nipah virus vaccines protect pigs against challenge. J. Virol. 2006, 80, 7929–7938.
- Yoneda, M.; Georges-Courbot, M.C.; Ikeda, F.; Ishii, M.; Nagata, N.; Jacquot, F.; Raoul, H.; Sato, H.; Kai, C. Recombinant measles virus vaccine expressing the Nipah virus glycoprotein protects against lethal Nipah virus challenge. PLoS ONE 2013, 8, e58414.
- Orusa, T.; Orusa, R.; Viani, A.; Carella, E.; Borgogno Mondino, E. Geomatics and EO data to support wildlife diseases assessment at landscape level: A pilot experience to map infectious keratoconjunctivitis in Chamois and phenological trends in Aosta Valley (NW Italy). Remote Sens. 2020, 12, 3542.
- Carella, E.; Orusa, T.; Viani, A.; Meloni, D.; Borgogno Mondino, E.; Orusa, R. An integrated, tentative remote-sensing approach based on NDVI entropy to model canine distemper virus in wildlife and to prompt science-based management policies. Animals 2022, 12, 1049.
- Kumar, V.B.; Rooney, N.; Carr, A. Nipah virus from bats—Another potential pandemic? Risk mapping the impact of anthropogenic and climate change on the transmission of Nipah virus infection to humans. medRxiv 2022.
- Welbergen, J.A.; Klose, S.M.; Markus, N.; Eby, P. Climate change and the effects of temperature extremes on Australian flying-foxes. Proc. Biol. Sci. 2008, 275, 419–425.
- Ratnayake, H.U.; Kearney, M.R.; Govekar, P.; Karoly, D.; Welbergen, J.A. Forecasting wildlife die-offs from extreme heat events. Anim. Conserv. 2019, 22, 386–395.
- Diengdoh, V.L.; Ondei, S.; Hunt, M.; Brook, B.W. Predicted impacts of climate change and extreme temperature events on the future distribution of fruit bat species in Australia. Glob. Ecol. Conserv. 2022, 37, e02181.
- European Union (EU). Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). Off. J. 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0429&from=EN (accessed on 22 December 2022).
- Aziz, B.J.; Azri, B.A. Nipah virus infection—Malaysia experience. In Proceedings of the World Organization of Animal Health (OIE) Conference WILDLIFE ACTES 2011; Available online: https://www.woah.org/fileadmin/Home/eng/Conferences_Events/sites/WILDLIFE_ACTES_2011/Presentations/S1_3_AzriBinAdzhar.pdf (accessed on 22 December 2022).
- National Centre for Disease Control (NCDC) India. Available online: https://ncdc.gov.in/ (accessed on 22 December 2022).
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Goldsmith, C.S. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435.
- Li, T.; Shen, Q.T. Insights into paramyxovirus nucleocapsids from diverse assemblies. Viruses 2021, 13, 2479.
- Middleton, D. Hendra virus. Vet. Clin. N. Am. Equine Pract. 2014, 30, 579–589.
- Middleton, D.; Pallister, J.; Klein, R.; Feng, Y.R.; Haining, J.; Arkinstall, R.; Frazer, L.; Huang, J.A.; Edwards, N.; Wareing, M.; et al. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis. 2014, 20, 372–379.
- Enchèry, F.; Horvat, B. Understanding the interaction between Henipaviruses and their natural host, fruit bats: Paving the way toward control of highly lethal infection in humans. Int. Rev. Immunol. 2017, 36, 108–121.
- Eaton, B.T.; Broder, C.C.; Middleton, D.; Wang, L.F. Hendra and Nipah viruses: Different and dangerous. Nat. Rev. Microbiol. 2006, 4, 23–35.
- Iehlé, C.; Razafitrimo, G.; Razainirina, J.; Andriaholinirina, N.; Goodman, S.M.; Faure, C.; Georges-Courbot, M.C.; Rousset, D.; Reynes, J.M. Henipavirus and Tioman virus antibodies in pteropodid bats, Madagascar. Emerg. Infect. Dis. 2007, 13, 159–161.
- Hayman, D.T.S.; Suu-Ire, R.; Breed, A.C.; McEachern, J.A.; Wang, L.; Wood, J.L.N.; Cunningham, A.A. Evidence of Henipavirus infection in West African fruit bats. PLoS ONE 2008, 3, e2739.
- Drexler, J.F.; Corman, V.M.; Gloza-Rausch, F.; Seebens, A.; Annan, A.; Ipsen, A.; Kruppa, T.; Müller, M.A.; Kalko, E.K.V.; Adu-Sarkodie, Y.; et al. Henipavirus RNA in African bats. PLoS ONE 2009, 4, e6367.
- Hayman, D.T.S.; Wang, L.F.; Barr, J.; Baker, K.S.; Suu-Ire, R.; Broder, C.C.; Cunningham, A.A.; Wood, J.L.N. Antibodies to Henipavirus or henipa-like viruses in domestic pigs in Ghana, West Africa. PLoS ONE 2011, 6, e25256.
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Cottontail, V.M.; Rasche, A.; Yordanov, S.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796.
- Mbu’u, C.M.; Mbacham, W.F.; Gontao, P.; Sado Kamdem, S.L.; Nlôga, A.M.N.; Groschup, M.H.; Wade, A.; Fischer, K.; Balkema-Buschmann, A. Henipaviruses at the interface between bats, livestock and human population in Africa. Vector-Borne Zoonotic Dis. 2019, 19, 455–465.
- Pernet, O.; Schneider, B.S.; Beaty, S.M.; LeBreton, M.; Yun, T.E.; Park, A.; Zachariah, T.T.; Bowden, T.A.; Hitchens, P.; Ramirez, C.M.; et al. Evidence for Henipavirus spillover into human populations in Africa. Nat. Commun. 2014, 5, 5342.
- Zhu, Z.; Dimitrov, A.S.; Bossart, K.N.; Crameri, G.; Bishop, K.A.; Choudhry, V.; Mungall, B.A.; Feng, Y.R.; Choudhary, A.; Zhang, M.Y.; et al. Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J. Virol. 2006, 80, 891–899.
- Bellini, W.J.; Harcourt, B.H.; Bowden, N.; Rota, P.A. Nipah virus: An emergent paramyxovirus causing severe encephalitis in humans. J. Neurovirol. 2005, 11, 481–487.
- Lamb, R.A.; Parks, G.D. Paramyxoviridae: The viruses and their replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2006; pp. 1449–1496.
- El Najjar, F.; Schmitt, A.P.; Dutch, R.E. Paramyxovirus glycoprotein incorporation, assembly and budding: A three way dance for infectious particle production. Viruses 2014, 6, 3019–3054.
- Maisner, A.; Neufeld, J.; Weingartl, H. Organ- and endotheliotropism of Nipah virus infections in vivo and in vitro. Thromb. Haemost. 2009, 102, 1014–1023.
- Aguilar, H.C.; Henderson, B.A.; Zamora, J.L.; Johnston, G.P. Paramyxovirus glycoproteins and the membrane fusion process. Curr. Clin. Microbiol. Rep. 2016, 3, 142–154.
- Walpita, P.; Cong, Y.; Jahrling, P.B.; Rojas, O.; Postnikova, E.; Yu, S.; Johns, L.; Holbrook, M.R. A VLP-based vaccine provides complete protection against Nipah virus challenge following multiple-dose or single-dose vaccination schedules in a hamster model. NPJ Vaccines 2017, 2, 21.
- Wang, L.; Harcourt, B.H.; Yu, M.; Tamin, A.; Rota, P.A.; Bellini, W.J.; Eaton, B.T. Molecular biology of Hendra and Nipah viruses. Microbes Infect. 2001, 3, 279–287.
- Harcourt, B.H.; Tamin, A.; Ksiazek, T.G.; Rollin, P.E.; Anderson, L.J.; Bellini, W.J.; Rota, P.A. Molecular characterization of Nipah virus, a newly emergent paramyxovirus. Virology 2000, 271, 334–349.
- Harcourt, B.H.; Lowe, L.; Tamin, A.; Liu, X.; Bankamp, B.; Bowden, N.; Rollin, P.E.; Comer, J.A.; Ksiazek, T.G.; Hossain, M.J.; et al. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg. Infect. Dis. 2005, 11, 1594–1597.
- de Wit, E.; Munster, V.J. Nipah virus emergence, transmission, and pathogenesis. In Global Virology I—Identifying and Investigating Viral Diseases; Shapshak, P., Sinnott, J., Somboonwit, C., Kuhn, J., Eds.; Springer: New York, NY, USA, 2015; pp. 125–146.
- de Wit, E.; Munster, V.J. Animal models of disease shed light on Nipah virus pathogenesis and transmission. J. Pathol. 2015, 235, 196–205.
- Angeletti, S.; Presti, A.L.; Cella, E.; Ciccozzi, M. Molecular epidemiology and phylogeny of Nipah virus infection: A mini review. Asian Pac. J. Trop. Med. 2016, 9, 630–634.
- AbuBakar, S.; Chang, L.Y.; Ali, A.R.; Sharifah, S.H.; Yusoff, K.; Zamrod, Z. Isolation and molecular identification of Nipah virus from pigs. Emerg. Infect. Dis. 2004, 10, 2228–2230.
- Liew, Y.J.M.; Ibrahim, P.A.S.; Ong, H.M.; Chong, C.N.; Tan, C.T.; Schee, J.P.; Gómez Román, R.; Cherian, N.G.; Wong, W.F.; Chang, L.Y. The immunobiology of Nipah virus. Microorganisms 2022, 10, 1162.
- Tan, C.T.; Chua, K.B. Nipah virus encephalitis. Curr. Infect. Dis. Rep. 2008, 10, 315–320.
- Vidal, S.; Curran, J.; Kolakofsky, D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J. Virol. 1990, 64, 239–246.
- Steward, M.; Vipond, I.B.; Millar, N.S.; Emmerson, P.T. RNA editing in Newcastle disease virus. J. Gen. Virol. 1993, 74, 2539–2547.
- Delenda, C.; Taylor, G.; Hausmann, S.; Garcin, D.; Kolakofsky, D. Sendai viruses with altered P, V, and W protein expression. Virology 1998, 242, 327–337.
- Yu, M.; Hansson, E.; Langedijk, J.P.; Eaton, B.T.; Wang, L.F. The attachment protein of Hendra virus has high structural similarity but limited primary sequence homology compared with viruses in the genus Paramyxovirus. Virology 1998, 251, 227–233.
- Reynes, J.M.; Counor, D.; Ong, S.; Faure, C.; Seng, V.; Molia, S.; Walston, J.; Georges-Courbot, M.C.; Deubel, V.; Sarthou, J.L. Nipah virus in Lyle’s flying foxes, Cambodia. Emerg. Infect. Dis. 2005, 11, 1042–1047.
- Chadha, M.S.; Comer, J.A.; Lowe, L.; Rota, P.A.; Rollin, P.E.; Bellini, W.J.; Ksiazek, T.G.; Mishra, A. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg. Infect. Dis. 2006, 12, 235–240.
- Bonaparte, M.I.; Dimitrov, A.S.; Bossart, K.N.; Crameri, G.; Mungall, B.A.; Bishop, K.A.; Choudhry, V.; Dimitrov, D.S.; Wang, L.F.; Eaton, B.T. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc. Natl. Acad. Sci. USA 2005, 102, 10652–10657.
- Negrete, O.A.; Levroney, E.L.; Aguilar, H.C.; Bertolotti-Ciarlet, A.; Nazarian, R.; Tajyar, S.; Lee, B. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 2005, 436, 401–405.
- Negrete, O.A.; Wolf, M.C.; Aguilar, H.C.; Enterlein, S.; Wang, W.; Mühlberger, E.; Su, S.V.; Bertolotti-Ciarlet, A.; Flick, R.; Lee, B. Two key residues in ephrinB3 are critical for its use as an alternative receptor for Nipah virus. PLoS Pathog. 2006, 2, e7.
- Liebl, D.J.; Morris, C.J.; Henkemeyer, M.; Parada, L.F. mRNA expression of ephrins and Eph receptor tyrosine kinases in the neonatal and adult mouse central nervous system. J. Neurosci. Res. 2003, 71, 7–22.
- Zimmer, M.; Palmer, A.; Köhler, J.; Klein, R. Ephb-ephrinB bi-directional endocytosis terminates adhesion allowing contact-mediated repulsion. Nat. Cell Biol. 2003, 5, 869–878.
- Bossart, K.N.; Tachedjian, M.; McEachern, J.A.; Crameri, G.; Zhu, Z.; Dimitrov, D.S.; Broder, C.C.; Wang, L.F. Functional studies of host-specific ephrin-B ligands as Henipavirus receptors. Virology 2008, 372, 357–371.
- Hassan, M.Z.; Sazzad, H.M.S.; Luby, S.P.; Sturm-Ramirez, K.; Bhuiyan, M.U.; Rahman, M.Z.; Islam, M.M.; Ströher, U.; Sultana, S.; Kafi, M.A.H.; et al. Nipah virus contamination of hospital surfaces during outbreaks, Bangladesh, 2013–2014. Emerg. Infect. Dis. 2018, 24, 15–21.
- Nikolay, B.; Salje, H.; Hossain, M.J.; Khan, A.K.M.D.; Sazzad, H.M.S.; Rahman, M.; Daszak, P.; Ströher, U.; Pulliam, J.R.C.; Kilpatrick, A.M.; et al. Transmission of Nipah virus—14 Years of investigations in Bangladesh. N. Engl. J. Med. 2019, 380, 1804–1814.
- Epstein, J.H.; Rahman, S.A.; Zambriski, A.; Halpin, K.; Meehan, G.; Jamaluddin, A.A.; Hassan, S.S.; Field, H.E.; Hyatt, A.D.; Daszak, P. Henipavirus Ecology Research Group. Feral cats and risk for Nipah virus transmission, Emerg. Infect. Dis. 2006, 12, 1178–1179.
- World Organization for Animal Health (OIE). Nipah Virus Infection (Wild Animals). Available online: https://www.woah.org/en/disease/nipah-virus-infection-wild-animals/ (accessed on 22 December 2022).
- World Organization for Animal Health (OIE). Henipaviruses (Nipah viruses) (Infection with). Available online: https://www.woah.org/app/uploads/2022/02/henipaviruses-nipah-viruses-infection-with.pdf (accessed on 22 December 2022).
- Eshaghi, M.; Tan, W.S.; Mohodin, T.B.; Yusoff, K. Nipah virus glycoprotein: Production in baculovirus and application in diagnosis. Virus Res. 2004, 106, 71–76.
- Weingartl, H.M. Hendra and Nipah viruses: Pathogenesis, animal models and recent breakthroughs in vaccination. Vaccine 2015, 5, 59–74.
- Ching, P.K.G.; de los Reyes, V.C.; Sucaldito, M.N.; Tayag, E.; Columna-Vingno, A.B.; Malbas, F.F., Jr.; Bolo, G.C., Jr.; Sejvar, J.J.; Eagles, D.; Playford, G.; et al. Outbreak of Henipavirus infection, Philippines, 2014. Emerg. Infect. Dis. 2015, 21, 328–331.
- Anyamba, A.; Chretien, J.P.; Britch, S.C.; Soebiyanto, R.P.; Small, J.L.; Jepsen, R.; Forshey, B.M.; Sanchez, J.L.; Smith, R.D.; Harris, R.; et al. Global disease outbreaks associated with the 2015-2016 El Niño event. Sci. Rep. 2019, 9, 1930.
- Orusa, T.; Borgogno Mondino, E. Exploring short-term climate change effects on rangelands and broad-leaved forests by free satellite data in Aosta Valley (Northwest Italy). Climate 2021, 9, 47.
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate change increases cross-species viral transmission risk. Nature 2022, 607, 555–562.
- Translated by Volken, E.; Brönnimann, S. 1884. Köppen, W. “Die Wärmezonen der Erde, nach der Dauer der heissen, gemässigten und kalten Zeit und nach der Wirkung der Wärme auf die organische Welt betrachtet” . Meteorol. Z. 2011, 20, 351–360.
- Mickleburgh, S.P.; Hutson, A.M.; Racey, P.A. Old World Fruit Bats—An Action Plan for Their Conservation; International Union for Conservation of Nature and Natural Resources (IUCN): Gland, Switzerland, 1992; ISBN 2-8317-0055-8. Available online: https://portals.iucn.org/library/efiles/documents/1992-034.pdf (accessed on 22 December 2022).
- Palmer, C.; Price, O.F.; Bach, C. Foraging ecology of the black flying fox (Pteropus alecto) in the seasonal tropics of the Northern Territory, Australia. Wildl. Res. 2000, 27, 169–178.
- Courts, S. Dietary strategies of old world fruit bats (Megachiroptera, Pteropodidae): How do they obtain sufficient protein? Mammal Rev. 1998, 28, 185–193.
- Giles, J.R.; Eby, P.; Parry, H.; Peel, A.J.; Plowright, R.K.; Westcott, D.A.; McCallum, H. Environmental drivers of spatiotemporal foraging intensity in fruit bats and implications for Hendra virus ecology. Sci. Rep. 2018, 8, 9555.
- Edson, D.; Field, H.; McMichael, L.; Jordan, D.; Kung, N.; Mayer, D.; Smith, C. Flying-fox roost disturbance and Hendra virus spillover risk. PLoS ONE 2015, 10, e0125881.
- McMichael, L.; Edson, D.; Smith, C.; Mayer, D.; Smith, I.; Kopp, S.; Meers, J.; Field, H. Physiological stress and Hendra virus in flying-foxes (Pteropus spp.), Australia. PLoS ONE 2017, 12, e0182171.
- Edson, D.; Peel, A.J.; Huth, L.; Mayer, D.G.; Vidgen, M.E.; McMichael, L.; Broos, A.; Melville, D.; Kristoffersen, J.; de Jong, C.; et al. Time of year, age class and body condition predict Hendra virus infection in Australian black flying foxes (Pteropus alecto). Epidemiol. Infec. 2019, 147, e240.
- Plowright, R.K.; Field, H.E.; Smith, C.; Divljan, A.; Palmer, C.; Tabor, G.; Daszak, P.; Foley, J.E. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc. Biol. Sci. 2008, 275, 861–869.
- McFarlane, R.; Becker, N.; Field, H. Investigation of the climatic and environmental context of Hendra virus spillover events 1994–2010. PLoS ONE 2011, 6, e28374.
- Páez, D.J.; Giles, J.; McCallum, H.; Field, H.; Jordan, D.; Peel, A.J.; Plowright, R.K. Conditions affecting the timing and magnitude of Hendra virus shedding across pteropodid bat populations in Australia. Epidemiol. Infec. 2017, 145, 3143–3153.
- Martin, G.; Yanez-Arenas, C.; Chen, C.; Plowright, R.K.; Webb, R.J.; Skerratt, L.F. Climate change could increase the geographic extent of Hendra virus spillover risk. EcoHealth 2018, 15, 509–525.
- Martin, G.; Yanez-Arenas, C.; Plowright, R.K.; Chen, C.; Roberts, B.; Skerratt, L.F. Hendra virus spillover is a bimodal system driven by climatic factors. EcoHealth 2018, 15, 526–542.
- Timmermann, A.; Oberhuber, J.; Bacher, A.; Esch, M.; Latif, M.; Roeckner, E. Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature 1999, 398, 694–697.
- Mpelasoka, F.; Hennessy, K.; Jones, R.; Bates, B. Comparison of suitable drought indices for climate change impacts assessment over Australia towards resource management. Int. J. Climatol. 2008, 28, 1283–1292.
- Chua, K.B.; Koh, C.L.; Hooi, P.S.; Wee, K.F.; Khong, J.H.; Chua, B.H.; Chan, Y.P.; Lim, M.E.; Lam, S.K. Isolation of Nipah virus from Malaysian flying-foxes. Microbes Infect. 2002, 4, 145–151.
- Nahar, N.; Asaduzzaman, M.; Mandal, U.K.; Rimi, N.A.; Gurley, E.S.; Rahman, M.; Garcia, F.; Zimicki, S.; Sultana, R.; Luby, S.P. Hunting bats for human consumption in Bangladesh. EcoHealth 2020, 17, 139–151.
- Latinne, A.; Saputro, S.; Kalengkongan, J.; Kowel, C.L.; Gaghiwu, L.; Ransaleleh, T.A.; Nangoy, M.J.; Wahyuni, I.; Kusumaningrum, T.; Safari, D.; et al. Characterizing and quantifying the wildlife trade network in Sulawesi, Indonesia. Glob. Ecol. Conserv. 2020, 21, e00887.
- Latinne, A.; Saputro, S.; Kalengkongan, J.; Kowel, C.L.; Gaghiwu, L.; Ransaleleh, T.A.; Nangoy, M.J.; Wahyuni, I.; Kusumaningrum, T.; Safari, D.; et al. Characterizing and quantifying the wildlife trade network in Sulawesi, Indonesia. Glob. Ecol. Conserv. 2020, 21, e00887.
