COVID-19 is an infective disease resulting in widespread respiratory and non-respiratory symptoms prompted by SARS-CoV-2 infection. Interaction between SARS-CoV-2 and host cell receptors prompts activation of pro-inflammatory pathways which are involved in epithelial and endothelial damage mechanisms even after viral clearance. Since inflammation has been recognized as a critical step in COVID-19, anti-inflammatory therapies, including both steroids and non-steroids as well as cytokine inhibitors, have been proposed. Early treatment of COVID-19 has the potential to affect the clinical course of the disease regardless of underlying comorbid conditions. Non-steroidal anti-inflammatory drugs (NSAIDs), which are widely used for symptomatic relief of upper airway infections, became the mainstay of early phase treatment of COVID-19.
- NSAIDs
- COVID-19
- SARS-CoV-2
- inflammation
- ketoprofen
1. Introduction
2. Pharmacological Agents with Selective Activity against SARS-CoV-2
2.1. Antivirals Targeting SARS-CoV-2
2.1.1. Entry Inhibitors
Bamlanivimab-Etesevimab
Casirivimab and Imdevimab
Sotrovimab
Tixagevimab-Cilgavimab
Other Anti-SARS-CoV-2 Monoclonal Antibodies
2.1.2. Inhibitors of Viral Proteases
Lopinavir/Ritonavir
Nirmatrelvir/Ritonavir
2.1.3. Inhibitors of Viral RNA Dependent RNA Polymerase (RdRp)
Remdesivir
Molnupiravir
2.1.4. Host-Oriented Therapies for SARS-CoV-2 Infection
3. Anti-Inflammatory Drugs in COVID-19
3.1. Corticosteroids Use in COVID-19 Patients
3.2. Non-Steroidal Anti-Inflammatory Drugs in the Early Stage of the Therapeutic Scenario of COVID-19
3.3. Ketoprofen Lysine Salt in the Therapeutic Scenario of SARS-CoV-2 Infection
3.3.1. Ketoprofen Lysine Salt Mechanism of Action
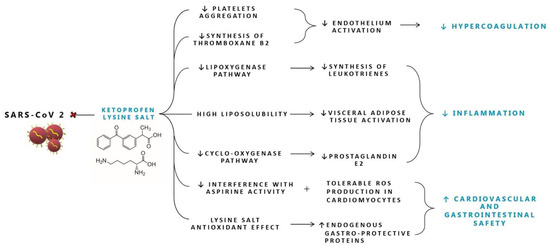
3.3.2. Ketoprofen Lysine Salt Cardiovascular Safety
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/molecules27248919
References
- Wang, Y.; Zhou, Y.; Yang, Z.; Xia, D.; Hu, Y.; Geng, S. Clinical Characteristics of Patients with Severe Pneumonia Caused by the SARS-CoV-2 in Wuhan, China. Respiration 2020, 99, 649–657.
- Wadman, J.C.-F.M.; Kaiser, C. How Does Coronavirus Kill? Clinicians Trace a Ferocious Rampage through the Body, from Brain to Toes. Science 2020.
- Zhou, Q.; Zhao, S.; Gan, L.; Wang, Z.; Peng, S.; Li, Q.; Liu, H.; Liu, X.; Wang, Z.; Shi, Q.; et al. Use of Non-Steroidal Anti-Inflammatory Drugs and Adverse Outcomes during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. EClinicalMedicine 2022, 46, 101373.
- Chambers, R.C.; Scotton, C.J. Coagulation Cascade Proteinases in Lung Injury and Fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 96–101.
- Kreuzberger, N.; Hirsch, C.; Chai, K.L.; Tomlinson, E.; Khosravi, Z.; Popp, M.; Neidhardt, M.; Piechotta, V.; Salomon, S.; Valk, S.J.; et al. SARS-CoV-2-Neutralising Monoclonal Antibodies for Treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 9, CD013825.
- O’Brien, M.P.; Forleo-Neto, E.; Sarkar, N.; Isa, F.; Hou, P.; Chan, K.-C.; Musser, B.J.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; et al. Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA 2022, 327, 432–441.
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950.
- Holland, T.L. Tixagevimab–Cilgavimab for Treatment of Patients Hospitalised with COVID-19: A Randomised, Double-Blind, Phase 3 Trial. Lancet Respir. Med. 2022, 10, 972–984.
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (Bebtelovimab) Potently Neutralizes SARS-CoV-2 Variants. Cell Rep. 2022, 39, 110812.
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799.
- National Institutes of Health. Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19). COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2019.
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 Polymerase Stalling by Remdesivir. Nat. Commun. 2021, 12, 279.
- Scialo, F.; Vitale, M.; Daniele, A.; Nigro, E.; Perrotta, F.; Gelzo, M.; Iadevaia, C.; Cerqua, F.S.; Costigliola, A.; Allocca, V.; et al. SARS-CoV-2: One Year in the Pandemic. What Have We Learned, the New Vaccine Era and the Threat of SARS-Cov-2 Variants. Biomedicines 2021, 9, 611.
- D’Agnano, V.; Scialò, F.; Perna, F.; Atripaldi, L.; Sanduzzi, S.; Allocca, V.; Vitale, M.; Pastore, L.; Bianco, A.; Perrotta, F. Exploring the Role of Krebs von Den Lungen-6 in Severe to Critical COVID-19 Patients. Life 2022, 12, 1141.
- NIH. COVID-19 Treatment Guidelines Therapeutic Management of Hospitalized Adults With COVID-19. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ (accessed on 11 December 2022).
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520.
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-Directed Therapies for Bacterial and Viral Infections. Nat. Rev. Drug Discov. 2018, 17, 35–56.
- Paschos, K.; Allday, M.J. Epigenetic Reprogramming of Host Genes in Viral and Microbial Pathogenesis. Trends Microbiol. 2010, 18, 439–447.
- Tripathi, D.; Sodani, M.; Gupta, P.K.; Kulkarni, S. Host Directed Therapies: COVID-19 and Beyond. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100058.
- Monk, P.D.; Marsden, R.J.; Tear, V.J.; Brookes, J.; Batten, T.N.; Mankowski, M.; Gabbay, F.J.; Davies, D.E.; Holgate, S.T.; Ho, L.-P.; et al. Safety and Efficacy of Inhaled Nebulised Interferon Beta-1a (SNG001) for Treatment of SARS-CoV-2 Infection: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Respir. Med. 2021, 9, 196–206.
- Laterre, P.F.; François, B.; Collienne, C.; Hantson, P.; Jeannet, R.; Remy, K.E.; Hotchkiss, R.S. Association of Interleukin 7 Immunotherapy With Lymphocyte Counts Among Patients With Severe Coronavirus Disease 2019 (COVID-19). JAMA Netw. Open 2020, 3, e2016485.
- Lee, J.S.; Shin, E.-C. The Type I Interferon Response in COVID-19: Implications for Treatment. Nat. Rev. Immunol. 2020, 20, 585–586.
- Jafari, A.; Esmaeilzadeh, Z.; Khezri, M.R.; Ghasemnejad-Berenji, H.; Pashapour, S.; Sadeghpour, S.; Ghasemnejad-Berenji, M. An Overview of Possible Pivotal Mechanisms of Genistein as a Potential Phytochemical against SARS-CoV-2 Infection: A Hypothesis. J. Food Biochem. 2022, 46, e14345.
- Olejnik, J.; Hume, A.J.; Mühlberger, E. Toll-like Receptor 4 in Acute Viral Infection: Too Much of a Good Thing. PLOS Pathog. 2018, 14, e1007390.
- Riedemann, T.; Patchev, A.V.; Cho, K.; Almeida, O.F.X. Corticosteroids: Way Upstream. Mol. Brain 2010, 3, 2.
- Perico, N.; Cortinovis, M.; Suter, F.; Remuzzi, G. Home as the New Frontier for the Treatment of COVID-19: The Case for Anti-Inflammatory Agents. Lancet. Infect. Dis. 2022.
- Duvignaud, A.; Lhomme, E.; Onaisi, R.; Sitta, R.; Gelley, A.; Chastang, J.; Piroth, L.; Binquet, C.; Dupouy, J.; Makinson, A.; et al. Inhaled Ciclesonide for Outpatient Treatment of COVID-19 in Adults at Risk of Adverse Outcomes: A Randomised Controlled Trial (COVERAGE). Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 28, 1010–1016.
- Ezer, N.; Belga, S.; Daneman, N.; Chan, A.; Smith, B.M.; Daniels, S.-A.; Moran, K.; Besson, C.; Smyth, L.Y.; Bartlett, S.J.; et al. Inhaled and Intranasal Ciclesonide for the Treatment of COVID-19 in Adult Outpatients: CONTAIN Phase II Randomised Controlled Trial. BMJ 2021, 375, e068060.
- EMA. EMA Gives Advice on the Use of Non-Steroidal Anti-Inflammatories for COVID-19. Eur. Med. Agency 2020, 1–4.
- Dorris, S.L.; Peebles, R.S.J. PGI2 as a Regulator of Inflammatory Diseases. Mediat. Inflamm. 2012, 2012, 926968.
- Zhao, H.; Huang, S.; Huang, S.; Liu, F.; Shao, W.; Mei, K.; Ma, J.; Jiang, Y.; Wan, J.; Zhu, W.; et al. Prevalence of NSAID Use among People with COVID-19 and the Association with COVID-19-Related Outcomes: Systematic Review and Meta-Analysis. Br. J. Clin. Pharmacol. 2022, 88, 5113–5127.
- Arienzo, D.M.; Pennisi, M.; Zanolo, G.; Borsa, M. Ketoprofen Lysine: Ketoprofen Serum Levels and Analgesic Activity. Drugs Under Exp. Clin. Res. 1984, 10, 2–3.
- McCormack, K.; Brune, K. Dissociation between the Antinociceptive and Anti-Inflammatory Effects of the Nonsteroidal Anti-Inflammatory Drugs. A Survey of Their Analgesic Efficacy. Drugs 1991, 41, 533–547.
- Sarzi-Puttini, P.; Atzeni, F.; Lanata, L.; Bagnasco, M.; Colombo, M.; Fischer, F.; D’Imporzano, M. Pain and Ketoprofen: What Is Its Role in Clinical Practice? Reumatismo 2010, 62, 172–188.
- Varrassi, G.; Alon, E.; Bagnasco, M.; Lanata, L.; Mayoral-Rojals, V.; Paladini, A.; Pergolizzi, J.V.; Perrot, S.; Scarpignato, C.; Tölle, T. Towards an Effective and Safe Treatment of Inflammatory Pain: A Delphi-Guided Expert Consensus. Adv. Ther. 2019, 36, 2618–2637.
- Rusca, A. Two Way Crossover, Randomised, Single Dose Comparative Bioavailability Study of Ketoprofen Lysine Salt after Oral Admin-Istration to Healthy Volunteers of Both Sexes. (IPAS–KETO–025–94), Data on File 1994. File 1994, 1–8.
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28.
- Marmo, E.; Ottavo, R.; Giordano, L.; Paone, G.; Falcone, O.; Spaziante, G.; Visone, C.; Campidonico, U. Experimental assessment of some pharmacodynamic features of ketoprofen lysine. Pain relief activity, antipyretic effects, anti-inflammatory activity, anti-platelet aggregation activity and interference with the biosynthesis of prostaglandins. Arch. Sci. Med. 1980, 137, 387–404.
- Saxena, A.; Balaramnavar, V.M.; Hohlfeld, T.; Saxena, A.K. Drug/Drug Interaction of Common NSAIDs with Antiplatelet Effect of Aspirin in Human Platelets. Eur. J. Pharmacol. 2013, 721, 215–224.
- Catella-Lawson, F.; Reilly, M.P.; Kapoor, S.C.; Cucchiara, A.J.; DeMarco, S.; Tournier, B.; Vyas, S.N.; FitzGerald, G.A. Cyclooxygenase Inhibitors and the Antiplatelet Effects of Aspirin. N. Engl. J. Med. 2001, 345, 1809–1817.
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840.
