Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Per- and polyfluoroalkyl substances (PFASs) are a family of highly toxic emerging contaminants that have caught the attention of both the public and private sectors due to their adverse health impacts on society. The widely adopted separation technologies can remove PFASs from being in contact with humans; however, they remain in the environment and continue to pose health risks. On the other hand, the destructive technologies can effectively destroy PFAS compounds and fully address society’s urgent need to remediate this harmful family of chemical compounds.
- per- and polyfluoroalkyl substances
- destruction technologies
- separation technologies
1. Introduction
Per- and polyfluoroalkyl substances (PFASs) have been designated as emerging contaminants of concern since the early 2000s [1][2][3][4]. PFAS compounds have been detected in surface and groundwater at more than 2000 locations in the US, with the highest concentrations at former Department of Defense (DOD) fire-training facilities [2][3][5][6][7]. PFASs comprise a diverse group of synthetic chemicals used for over 90 years [8]. It is a complex group of chemicals, which consists of compounds with carbon-fluorine solid bonds (C-F) and is the shortest and strongest known covalent bond in nature and is responsible for the thermal and chemical stability of PFASs [9][10][11][12][13]. In conjunction with their ability to act as surfactants, these properties make PFASs ideal for a wide range of industrial and commercial applications [1].
Due to the low surface tension and wetting properties of PFAS, they are used in paint additives, non-stick cookware, and firefighting foams [9][14][15]. Unfortunately, these same properties also rendered PFASs bio-accumulative, toxic to both the environment and human health, and ubiquitous in the environment [16][17]. Perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) have been the most extensively manufactured and hence most frequently detected PFASs in the environment [18][19][20][21][22][23][24]. Figure 1 shows the chemical structure, the three-dimensional view, the tail group (C-F bonds), and the head groups of the carboxylates and sulfonics of these two molecules, PFOA and PFOS. Significant sources of PFAS released to the environment include fire training/fire response sites, industrial sites, landfills, and wastewater treatment plants/biosolids [25].
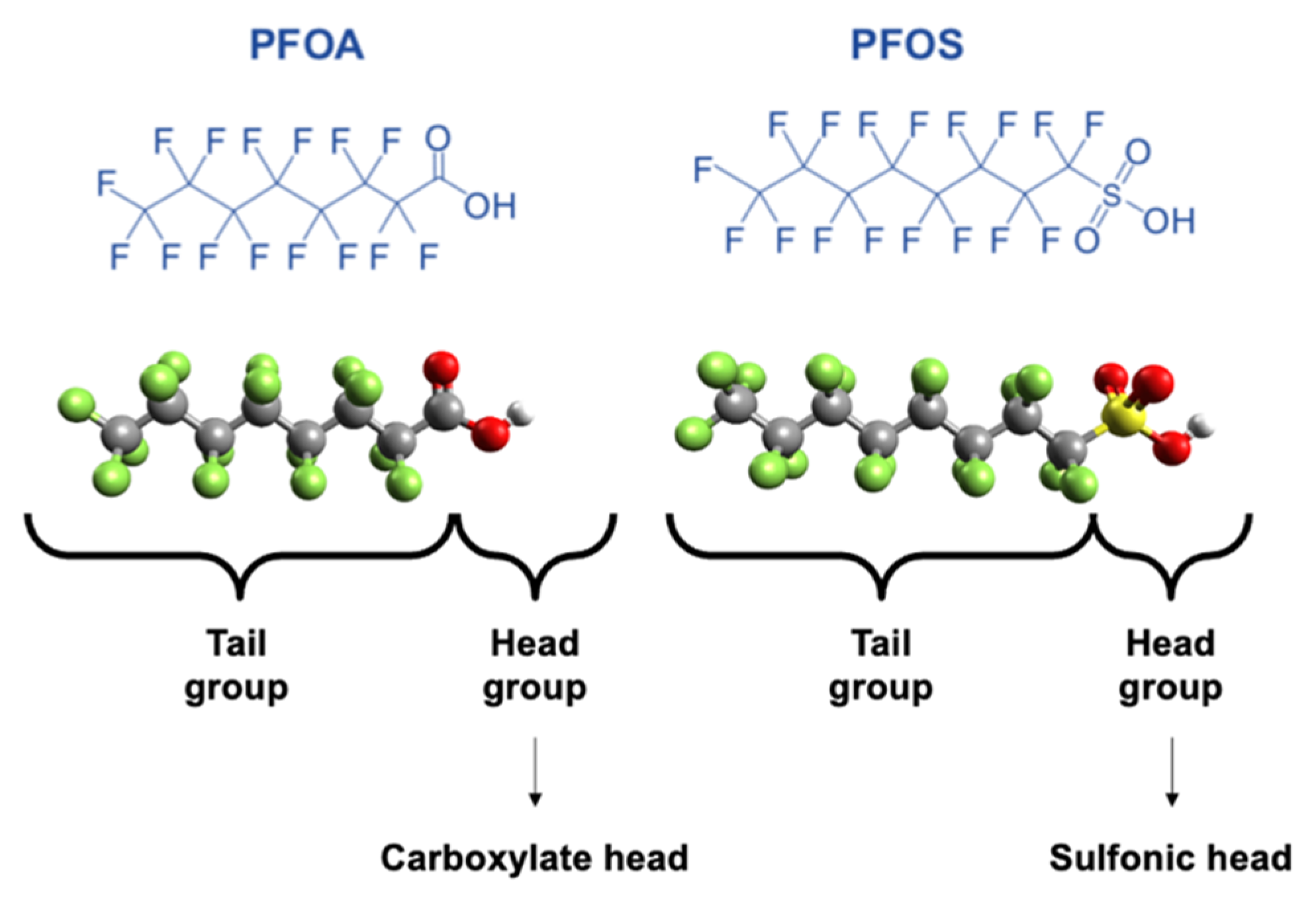
Figure 1. PFOA and PFOS chemical structures.
The most widely used non-polymer PFASs are perfluorooctanoic acid (PFOA), perfluorooctanoic sulfonic acid (PFOS), and perfluorohexane sulfonic acid (PFHxS) [26][27]. For the past five decades, mixtures of PFAS were added as film-formers and foam stabilizers in aqueous film-forming foams (AFFFs) and film-forming fluoroproteins (FFFPs). They have been used as fuel repellents to extinguish flammable aircraft fuel fires in the aeronautics industry [28][29][30]; however, PFAA precursors found in AFFF are also converted over time into PFASs and have lasted for decades in the environment [15][31]. PFASs have been detected in the local streams, soil, plants, and animal tissues throughout these sites. The presence of PFAS food packaging has been cited as an important pathway for human exposure other than direct consumption of PFAS-contaminated water. Studies in humans have discovered that PFASs can lead to cancer, problems in kidney function, metabolic disruption, and many other health issues.
The release of PFASs into the environment has become a growing concern of national and regional regulatory agencies due to the resistance of PFASs to natural degradation and their persistence in animals, humans, and the environment [27][32][33]. For this reason, use restrictions and other regulations for PFASs have been implemented around the world. The United States Environmental Protection Agency (USEPA) has developed advisory limits on PFASs in drinking water since 2009. The most recent guideline implemented in June 2022 has advisory limits of 0.004 parts per trillion (ppt) of PFOA, 0.02 ppt of PFOS, 10 ppt of GenX chemicals, and 2000 ppt of PFBS. In May 2022, the USEPA added five more PFAS compounds for site cleanups (perfluoronanoic acid (PFNA), PFHxS, perfluorononanoate, perfluorooctanoate, and perfluorohexanesulfonate) based on risk-based values for regional screening levels (RSLs).
Conventional water and wastewater treatment facilities are not capable of removing PFASs [15]. The PFAS effluent concentrations can be significantly higher when compared to influent levels due to PFAS-like precursor compounds breaking down within such treatment systems [30][34][35][36][37][38][39][40][41]. Therefore, numerous studies have developed technologies to capture these pollutants in drinking water sources. These technologies include ion exchange resin (IXR), granular activated carbon (GAC), nanofiltration (NF), and reverse osmosis (RO). Even though removal technologies have been proven effective in PFAS separation or adsorption, they do not eliminate or destroy PFASs. These are only interim actions involving the physical mass transfer (sequestration) of PFASs.
Separation technologies such as IXR and GAC can momentarily remove the PFAS from a specific medium; however, they remain in the environment and continues to cause health risks. GAC and IXR are currently the most common or widely accepted treatment options for PFAS removal from groundwater and drinking water. The IXR systems are more expensive than GAC, but IXR applications are gaining popularity over GAC due to the higher adsorption capacities of IXR, as well as shorter contact times and smaller equipment footprints. More importantly, IXR can be regenerated on-site to a nearly virgin capacity and hence can be used repeatedly, whereas the on-site regeneration of GAC is not feasible. The brine solution or fractions (still bottom-SB) resulting from IXR regeneration contains high concentrations of PFASs (typically in ranges of ppm), salt, and residual organic compounds. Worldwide, the high concentrations of PFASs generated from IXR technology are currently stored in secure sites until suitable destruction technology is identified. Hence the purpose of this publication is to facilitate the discussion on the selection of the best available destruction technology for the high concentrations of PFASs generated from IXR technology for the complete destruction of PFAS compounds.
Even though destructive technologies are still in the development stage, they have shown great promise to destroy PFAS compounds and provide a solution to effectively address society’s urgent need to remediate this harmful family of chemical compounds. Some of the technologies presented here are still under development at the lab scale, while others have already been tested in the field.
The following technologies are discussed herein:
- Electrochemical oxidation;
- Plasma;
- Photocatalysis;
- Sonolysis;
- Supercritical water oxidation;
- Thermal degradation/incineration.
Please note that despite extensive published experimental and numerical research on the degradation or mineralization of PFASs, there are still major gaps in the experimental data, which makes it challenging to perform a complete evaluation of the efficiency of those technologies. There are several unanswered questions to obtain a clear picture to allow the technology to be optimized.
There is an urgent societal need to investigate cost-effective and safe PFAS mineralization technologies with minimal adverse environmental impacts. Hence this topic is extremely relevant as it will help the user to understand the mechanics of PFAS destruction technologies at the development stage and will allow optimization of those to have a cleaner environment free of PFAS contaminants.
2. Electrochemical Oxidation
The electrochemical oxidation (EO) method oxidizes and reduces organic pollutants by applying an electrical current through a conductive solution between an anode and a cathode. The contaminants are either adsorbed and degraded at the electrode or in the liquid medium [15][42]. The EO method has been shown to break PFASs into environmentally benign products through direct and indirect reactions. The direct oxidation results from electron transfer from the PFAS compound to the anode. At the same time, indirect mechanisms involve the creation of powerful oxidants known as radicals electrochemically [43][44][45]. A series of reactions separate intermediate products from the parent compound and are subsequently defluorinated [46][47][48]. The treatment of long-chain PFAS has demonstrated removals as high as >99%. On the other hand, short-chain PFASs are generally more challenging for the EO method to degrade [30], and it can even increase the PFAS concentration after treatment due to the conversion of precursors [44][49][50]. The EO mechanism used for PFAS destruction can be observed in Figure 2.
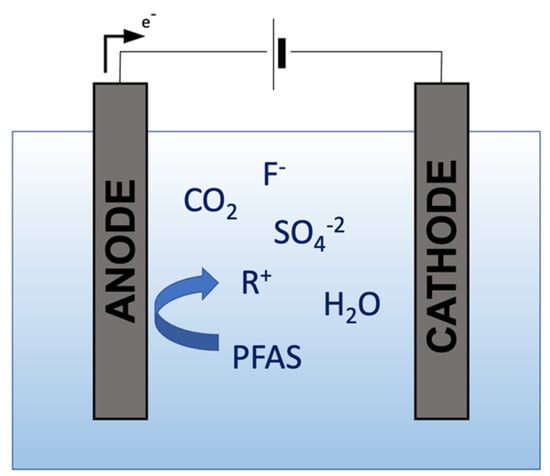
Figure 2. Mechanism of Electrochemical Oxidation.
The time of treatment of PFAS via EO depends on a wide range of variables such as electrode characteristics and surface area, initial PFAS concentration (presence of co-contaminants), efficiency target, and voltage. Recent studies have identified the use of reactive EO membranes can significantly expedite the reaction time [51]. Currently, most of the experiments are performed in laboratory-generated waste streams [45]. The real-world PFAS waste will likely need an extended treatment time or reduced performance and a decrease in the electrode lifetimes [52]. The reader is referred to Veciana [30], who compares recently published PFAS treatment with EO using real contaminated samples.
According to an evaluation in the literature [50], the kinetics rates of PFAS EO degradation were optimized by increasing the applied electrical current densities in the range of 20–350 A/m2. Evaluation of current density efficiency showed that 50 mA/cm2 produced better efficiency on PFAS degradation when compared to 10 and 20 mA/cm2, presenting removal of PFBA > 95% and perfluoroalkyl acid (PFAAs) 99% within 8 h of treatment of synthetic PFAS samples [49]. The treatment time and energy consumption can be significantly reduced when working with high concentrated PFAS streams. One method for increasing the concentration would be to use a coupled system containing the EO electrodes and a separation technique such as ion exchange IXR, nanofiltration (NF), or membrane filtration. These treatment trains have been proven to be extremely effective and, indeed, can potentially decrease energy consumption by more than 50% [30][52][53][54]. Specialized electrodes with tracer metals acting as catalysts have shown superior performance.
3. Plasma
A plasma is an electrically charged gas created when adding energy which induces ionization of the gas molecules [27]. In plasma-based water treatment, highly reactive oxidative and reductive species are formed in response to the electrical discharge formed between two electrodes in the vicinity of liquid water [55][56][57]. The electrical discharge and its liquid interaction are shown in Figure 3, which conveys the mechanism behind the plasma technology. Additionally, temperature increases in the proximity of the discharge, the generation of shockwaves, and UV light emission occurs inside the reactor. Non-thermal plasma (NTP) is preferable for treating water contaminated with PFASs because NTP consumes a low level of energy at atmospheric pressure in air or with supporting gasses (He, Ne, Ar, O2 and N2) [58], showing higher excitation selectivity and energy efficiency than that for thermal plasma [15][59].
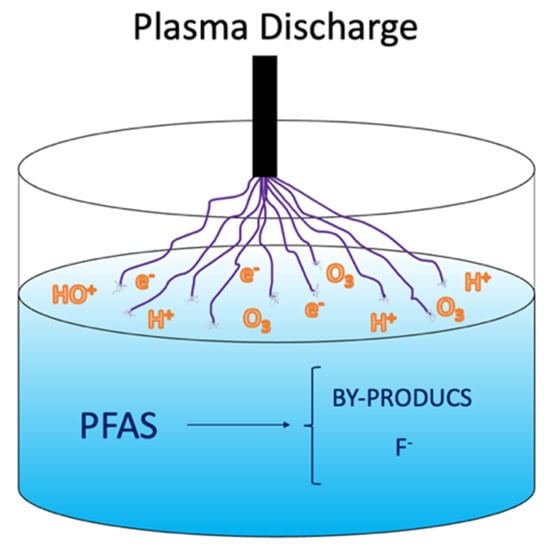
Figure 3. Non-thermal plasma approach for PFAS mineralization.
The NTP can be generated via diverse methods, including spark discharge, corona discharge, glow discharge, dielectric barrier discharge, and gliding arc discharge. The electrons are energized, and their temperature will be much greater than the gases in the environment. Consequently, the electrons will constantly collide with the gas’s atoms, generating electrons, radicals, ions, and photons [15][59]. The PFAS molecules are adsorbed onto the interface of the water bubbles, where the positively charged or negatively charged section of the PFAS collides with the ions with the highest energy in the plasma state [15][60][61]. Since the plasma discharge is a complete process, no additional chemicals are required to perform the treatment [58]. Argon bubbling has been reported to be the best-performing plasma reactor for treating surfactant-like compounds such as PFAAs [57][62][63]. The efficiency of plasma technology varies widely. It depends on several factors, for example, the reactor, electrode material, conductivity, applied voltage, PFAS type, pulse repetition rate, energy input, pH of the solution, liquid and gas temperature, liquid conductivity, and gas input [48].
4. Photocatalysis
Photocatalysis is a technology where a substance is activated due to the adsorption of a photon and in the presence of a photocatalyst which will accelerate the destruction reaction rate [64]. The photocatalyst substances are generally semiconductors. Recently, photocatalysts have been increasingly used because of their potential applications in solar energy conversion and environmental purification. Photocatalysis has enormous potential to treat organic contaminants in water and air [65]. The photocatalysis technology is an advanced oxidation process (AOP); consequently, it is applicable for the oxidation of a wide range of organic contaminants [65]. Heterogeneous photocatalysis substances have shown to be the most applicable due to their efficiency in degrading recalcitrant organic compounds, with similar characteristics as PFAS [65]. In past years, several studies have used heterogeneous photocatalysis oxidation to degrade recalcitrant organic compounds. The mechanism is achieved by the acceleration of a photoreaction in the presence of a catalyst [66]. Several catalysts such as In2O3, Fe2O3, TiO2, ZnO, CdS, and Ga2O3 can work as photocatalysts. At the present moment, titanium dioxide (TiO2) has been the compound that is widely investigated due to its ability to degrade organic pollutants and achieve satisfactory mineralization at a relatively low cost when compared to other destructive technologies [67].
The photodegradation can be performed with a wide range of wavelengths, which overcomes the problem that it is difficult to destroy the C-F bond by direct photolysis [65][68][69]. However, after the photocatalyst absorbs light energy, it can generate negatively charged electrons and positively charged hole pairs, which will move onto the surface of the photocatalyst and react with the adsorbed PFAS [70]. Figure 4 shows the PFAS destruction using photocatalysis, which is a staged or sequential reaction. As can be observed in Figure 4, the first cycle eliminates one carbon and two fluorine atoms in the PFAS molecule, and subsequent cycles will further reduce the PFAS chain length until it fully degrades the PFAS molecule. Identified ways to enhance the photocatalysis efficiency and cost by adding carbon materials enhanced the range of the frequency of light absorbed by photocatalysts, with the possibility of applying sunlight as a sustainable energy source [70][71]. Furthermore, modifications to these semiconductor-based chemical compositions of photocatalysts, their morphology, and their size can significantly improve PFAA removal and mineralization [15]. In addition, the photocatalytic and hydrophilic properties of TiO2 make it close to an ideal catalyst due to its high reactivity, reduced toxicity, chemical stability, and lower costs [72].
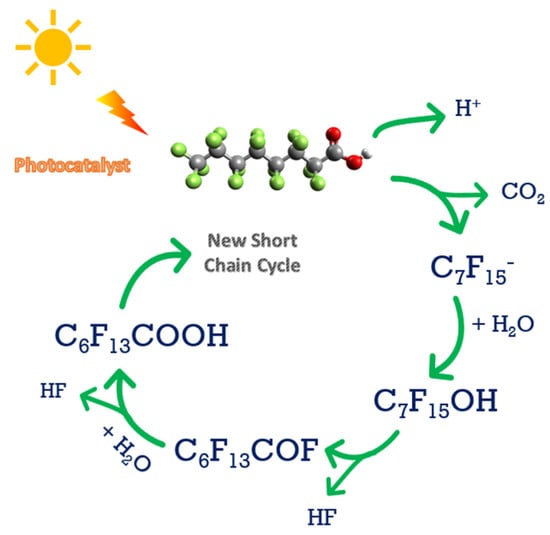
Figure 4. Photocatalytic method for PFAS degradation.
5. Sonolysis
Sonochemistry uses an acoustic field to create chemical reactions in a solution. The mechanism consists of the generation and implosion of vapor bubbles. Organic pollutant degradation is achieved by a combination of radical reaction and combustion by pyrolysis [73][74][75]. The bubble collapse is the driving mechanism responsible for pyrolysis and combustion of organic compounds in the vicinity of the imploding bubble. The imploding bubbles produce very high temperatures (average 5000 K) [76][77]. During pyrolysis, due to extreme heat, water vapor will also be converted to H− and OH− radicals. These radicals react and degrade organic contaminants [78]. This technology has been widely used to degrade organic compounds, including PFCs, where the decomposition occurs at the bubble/water interface due to pyrolysis [4][13][79]. Figure 5 shows a schematic view of the PFAS destruction with sonolysis. The PFAS molecules adsorbed into the bubble surface will be pyrolyzed to several products during bubble implosion, as shown in Figure 5.
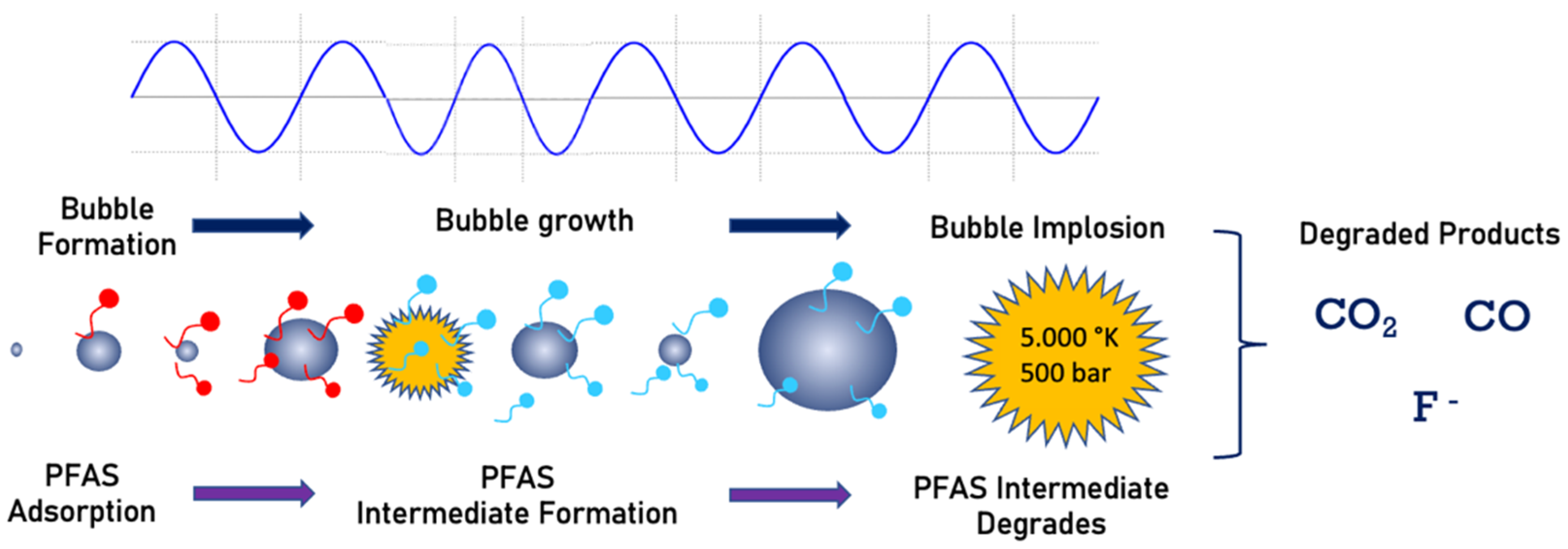
Figure 5. Sonolysis process of PFAS mineralization.
Research on sonochemical parameters indicates that ultrasonic frequency is highly important for the effective degradation of PFASs [75][80][81][82]. Although chemical activity can be further enhanced using a combination of frequencies [83][84], the production of cavitational bubble volume fractions is higher for dual-frequency than single-frequency reactors [84][85][86]. Along with frequency, power is also an important parameter of sonochemical degradation. Increasing the power increases the number of collapsed cavities formed, the maximum collapse temperature, and the sonochemical activity [87][88][89].
6. Supercritical Water Oxidation (SCWO)
Supercritical water oxidation (SCWO) is an oxidation treatment process where OM is transformed into water, carbon dioxide, and a few other products depending on the wasted stream being treated. The process can treat an extensive range of wet wastes without dewatering. The SCWO technology has already proven to be effective in the destruction of toxic and persistent organic contaminants such PFASs [90].
A supercritical water solution is a substance kept at a specific temperature and pressure above its critical point. Water above 374 °C and 218 atm becomes supercritical, a state where organic solubility significantly increases, and oxidation is enhanced [91][92]. Figure 6 shows the region where the water is in the supercritical state. It can be observed that, at the supercritical state, the liquid and vapor phases share similar properties. The fluid is neither a liquid nor a gas at this state and has properties of both states. Under these conditions, the fluid molecules start to behave differently. Supercritical water is highly expandable and compressible, and the mass transfer is unrestricted without distinct liquid and gas phases, promoting chemical reactions. Supercritical oxidation can break down compounds, such as PFASs, that do not oxidize at standard temperatures and pressures [91].
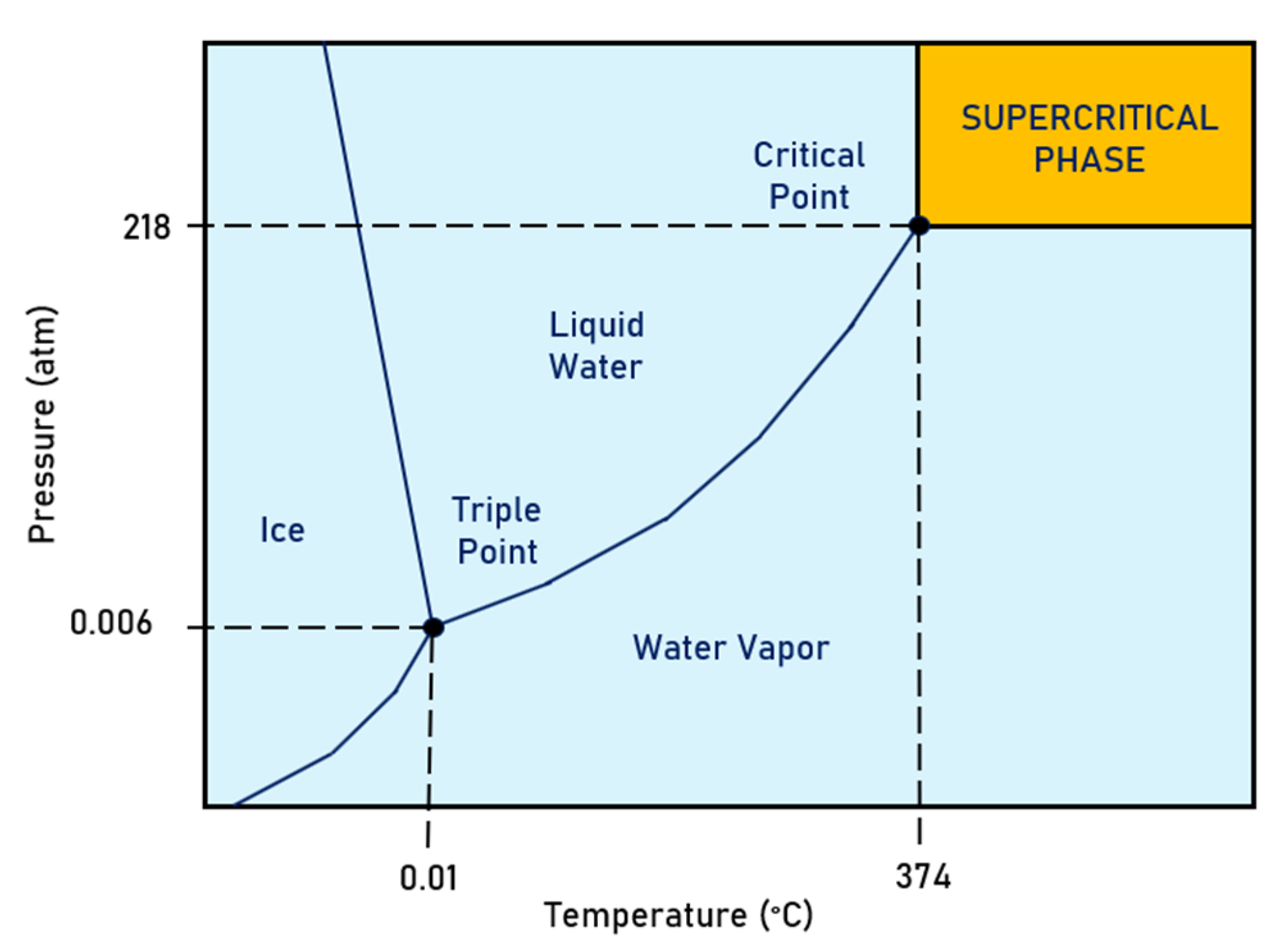
Figure 6. Water phase diagram highlighting the supercritical water region.
7. Thermal Degradation/Incineration
Heat is applied in thermal treatment to treat and decompose/destroy materials. This is a complicated process, and the mechanism depends on many factors, including the operating conditions such as temperature, the environment of the heating chamber, gases present, residence time, mixture composition of waste streams, and chemical characteristics of materials to be treated. As for PFAS incineration, under ideal conditions (i.e., mineralization), the destruction of PFASs results in final products such as carbon monoxide, carbon dioxide, water, hydrogen fluoride, and sulfur molecules or sulphuric acid in the case of sulfur-containing PFASs. Due to their unique structure, PFAS are considered chemically and thermally stable, which translates into a requirement to apply higher temperatures and long residence time to achieve a satisfactory level of destruction. Figure 7 shows the PFAS destruction path through incineration. Incineration is considered to be a thermal oxidation treatment method, and it is carried out in an oxygen-rich atmosphere at high temperatures. Most hazardous waste incinerators operate from 980 °C to 1200 °C [93]. Since the thermal destruction of most organic compounds occurs between 590 °C and 650 °C, the expectation is that nearly total destruction of the organics in the waste will be achieved, including PFAS [93].
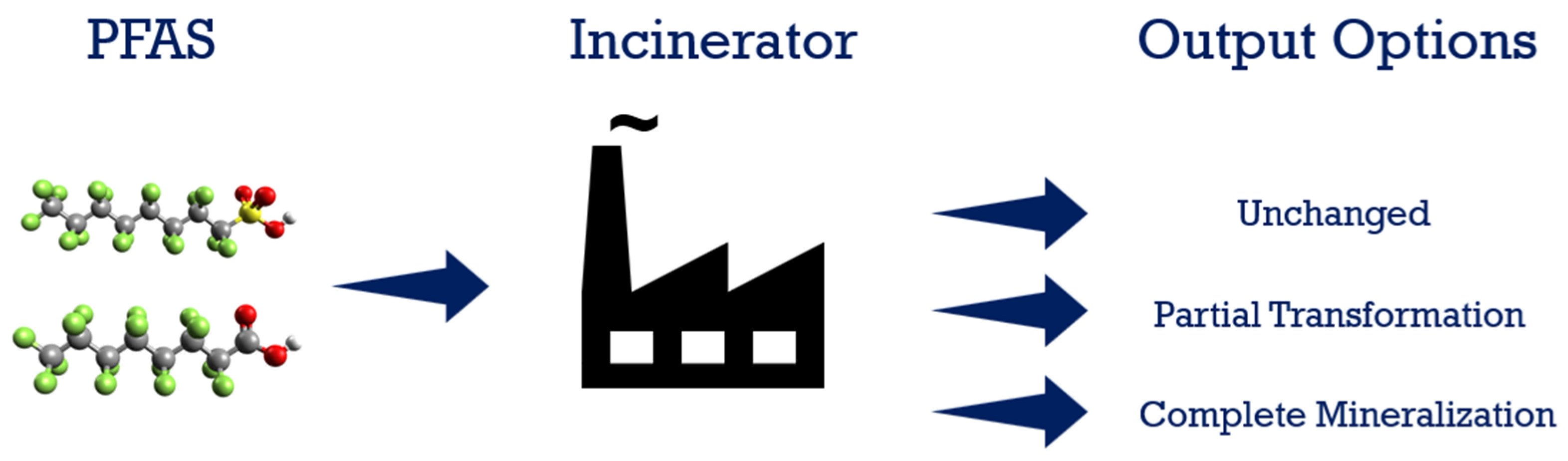
Figure 7. PFAS incineration.
In 2020, the U.S. EPA [94] published a technical brief on the incineration of PFAS with the main conclusion that the effectiveness of incineration in destroying PFAS and their fate in terms of potential mixed fluorinated organic byproduct formation is not clearly understood. A significant concern is that incomplete destruction of PFAS can result in the formation of PIC (products of incomplete combustion), e.g., smaller PFAS molecules, which could be a potential hazard. Only a few studies are available related to PFAS incineration in full-scale operating facilities [41][95][96][97]. According to Solo-Gabriele et al., increasing incinerator temperatures decreased the total treated PFAS concentrations. However, not all PFAS species decreased with increasing temperatures [95]. There is an alarming report of higher concentrations of PFOA found in the air at the incinerator sites compared to upwind sites [96]. Public concern is that the incineration may spread PFAS and not break them down. This publication claims that the preliminary data show that soil and surface water near a commercial facility in Cohoes, New York, that has burned firefighting foam containing PFAS are contaminated with PFAS [98].
PFAS incineration can occur directly for PFAS-based materials, such as firefighting foams or indirectly via the incineration of waste containing PFAS, such as textiles, etc. [99]. Recently the Defense Department issued a ban on incinerating PFAS-laden items, with particular emphasis on the aqueous film-forming foam often used in training and combat situations [100]. In addition, under the 2022 National Defense Authorization Act [101], the military is now prohibited from incinerating PFAS-containing materials in accordance with the Clean Air Act [102]. Most incineration studies monitored a limited number of compounds, leaving the question of “unmonitored” PFAS unanswered [103]. Even though multiple studies were done on the thermal degradation of PFAS [104][105][106][107][108], only limited data [109][110] is available on directly detecting degradation products during field-scale incineration. The main obstacle is still the lack of both suitable emission sampling methods (including industrial field sampling) to capture PFAS compounds and analytical methods to identify/detect PFAS and their thermal decomposition byproducts. The question remains unanswered as to how significant is the portion of volatile species that escape the analysis.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph192416397
References
- 3M Company. Fluorochemical Use, Distribution and Release Overview; 3M Company: Saint Paul, MN, USA, 1999.
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541.
- Jansen, K. ‘Forever chemicals’ no more? CEN Glob. Enterp. 2019, 97, 28–32.
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A review of the applications, environmental release, and remediation technologies of per- and polyfluoroalkyl substances. Int. J. Environ. Res. Public Health 2020, 17, 8117.
- ITRC; PFAS Team. Aqueous Film-Forming Foam (AFFF) Continued; ITRC: Washington, DC, USA, 2020.
- ITRC; PFAS Team. Per- and Polyfluoroalkyl (PFAS), Technical/Regulatory Guidance. Available online: https://pfas-1.itrcweb.org/wp-content/uploads/2020/10/itrc_pfas_techreg_sept_2020_508-1.pdf (accessed on 19 February 2022).
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of select perfluoroalkyl substances at U.S. air force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678–685.
- Ebnesajjad, S. Discovery and history of fluoropolymers. In Introduction to Fluoropolymers; Elsevier: Amsterdam, The Netherlands, 2013; pp. 17–35.
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K. Fluorinated alternatives to long-chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and their potential precursors. Environ. Int. 2013, 60, 242–248.
- Hekster, F.M.; Laane, R.W.P.M.; de Voogt, P. Environmental and toxicity effects of perfluoroalkylated substances. Rev. Environ. Contam. Toxicol. 2003, 99–121.
- Kissa, E. Fluorinated Surfactants and Repellents, 2nd ed.; Marcel Dekker: New York, NY, USA, 2001; Volume 97.
- ITRC; PFAS Team. Naming Conventions for Per-and Polyfluoroalkyl Substances (PFAS); ITRC: Washington, DC, USA, 2020.
- Meegoda, J.; Kewalramani, J.; Marsh, R.; de Souza, B.B.; Dahanayake, M. Per-and polyfluoroalkyl substances. Polym. Sci. 2021. Available online: https://encyclopedia.pub/entry/6459 (accessed on 9 September 2022).
- Vierke, L.; Staude, C.; Biegel-Engler, A.; Drost, W.; Schulte, C. Perfluorooctanoic Acid (PFOA)-main concerns and regulatory developments in Europe from an environmental point of view. Environ. Sci. Eur. 2012, 24, 16.
- Leung, S.C.E.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.T.; Li, Q. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total Environ. 2022, 827, 153669.
- Krafft, M.P.; Riess, J.G. Per- and polyfluorinated substances (PFASs): Environmental challenges. Curr. Opin. Colloid Interface Sci. 2015, 20, 192–212.
- Ojo, A.F.; Peng, C.; Ng, J.C. Assessing the human health risks of per- and polyfluoroalkyl substances: A need for greater focus on their interactions as mixtures. J. Hazard Mater. 2021, 407, 124863.
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342.
- Brusseau, M.L.; Anderson, R.H.; Guo, B. PFAS Concentrations in Soils: Background Levels versus Contaminated Sites. Sci. Total Environ. 2020, 740, 140017.
- Rankin, K.; Mabury, S.A.; Jenkins, T.M.; Washington, J.W. A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence. Chemosphere 2016, 161, 333–341.
- Ahrens, L.; Xie, Z.; Ebinghaus, R. Distribution of perfluoroalkyl compounds in seawater from Northern Europe, Atlantic Ocean, and Southern Ocean. Chemosphere 2010, 78, 1011–1016.
- Kwok, K.Y.; Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Murphy, M.B.; Horii, Y.; Petrick, G.; Kallerborn, R.; Kannan, K.; Murano, K.; et al. Transport of perfluoroalkyl substances (PFAS) from an Arctic glacier to downstream locations: Implications for sources. Sci. Total Environ. 2013, 447, 46–55.
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648.
- RIDOH; PFAS. Contamination of Water: Department of Health. Available online: https://health.ri.gov/water/about/pfas/ (accessed on 3 February 2022).
- ITRC; PFAS Team. PFAS. Available online: https://itrcweb.org/teams/active/pfas (accessed on 19 February 2022).
- Ma, D.; Zhong, H.; Lv, J.; Wang, Y.; Jiang, G. Levels, distributions, and sources of legacy and novel per- and perfluoroalkyl substances (PFAS) in the topsoil of Tianjin, China. J. Environ. Sci. 2022, 112, 71–81.
- Kazwini, T.; Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated review on emerging technologies for PFAS contaminated water treatment. Chem. Eng. Res. Des. 2022, 182, 667–700.
- Scheffey, J.L.; Darwin, R.L.; Leonard, J.T. Evaluating firefighting foams for aviation fire protection. Fire Technol. 1995, 31, 224–243.
- Cousins, I.T.; Vestergren, R.; Wang, Z.; Scheringer, M.; McLachlan, M.S. The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater. Environ. Int. 2016, 94, 331–340.
- Veciana, M.; Bräunig, J.; Farhat, A.; Pype, M.-L.; Freguia, S.; Carvalho, G.; Keller, J.; Ledezma, P. Electrochemical oxidation processes for PFAS removal from contaminated water and wastewater: Fundamentals, gaps and opportunities towards practical implementation. J. Hazard. Mater. 2022, 434, 128886.
- Houtz, E.F.; Higgins, C.P.; Field, J.A.; Sedlak, D.L. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 2013, 47, 8187–8195.
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review. Chem. Eng. J. 2017, 328, 927–942.
- Holmquist, H.; Fantke, P.; Cousins, I.T.; Owsianiak, M.; Liagkouridis, I.; Peters, G.M. An (eco)toxicity life cycle impact assessment framework for per- and polyfluoroalkyl substances. Environ. Sci. Technol. 2020, 54, 6224–6234.
- Coggan, T.L.; Moodie, D.; Kolobaric, A.; Szabo, D.; Shimeta, J.; Crosbie, N.D.; Lee, E.; Fernandes, M.; Clarke, B.O. An investigation into per- and polyfluoroalkyl substances (PFAS) in nineteen australian wastewater treatment plants (WWTPs). Heliyon 2019, 5, e02316.
- Gomez-Ruiz, B.; Gómez-Lavín, S.; Diban, N.; Boiteux, V.; Colin, A.; Dauchy, X.; Urtiaga, A. Efficient electrochemical degradation of poly- and perfluoroalkyl substances (PFASs) from the effluents of an industrial wastewater treatment plant. Chem. Eng. J. 2017, 322, 196–204.
- Dauchy, X.; Boiteux, V.; Bach, C.; Colin, A.; Hemard, J.; Rosin, C.; Munoz, J.F. Mass flows and fate of per- and polyfluoroalkyl substances (PFASs) in the wastewater treatment plant of a fluorochemical manufacturing facility. Sci. Total Environ. 2017, 576, 549–558.
- Schultz, M.M.; Higgins, C.P.; Huset, C.A.; Luthy, R.G.; Barofsky, D.F.; Field, J.A. Fluorochemical mass flows in a municipal wastewater treatment facility. Environ. Sci. Technol. 2006, 40, 7350–7357.
- Sinclair, E.; Kannan, K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1408–1414.
- Fuertes, I.; Gómez-Lavín, S.; Elizalde, M.P.; Urtiaga, A. Perfluorinated alkyl substances (PFASs) in Northern Spain municipal solid waste landfill leachates. Chemosphere 2017, 168, 399–407.
- Ahrens, L. Polyfluoroalkyl compounds in the aquatic environment: A review of their occurrence and fate. J. Environ. Monit. 2011, 13, 20–31.
- Loganathan, B.G.; Sajwan, K.S.; Sinclair, E.; Senthil Kumar, K.; Kannan, K. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res. 2007, 41, 4611–4620.
- Radjenovic, J.; Sedlak, D.L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ. Sci. Technol. 2015, 49, 11292–11302.
- Trautmann, A.M.; Schell, H.; Schmidt, K.R.; Mangold, K.-M.; Tiehm, A. Electrochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in groundwater. Water Sci. Technol. 2015, 71, 1569–1575.
- Liang, S.; Mora, R.; Huang, Q.; Casson, R.; Wang, Y.; Woodard, S.; Anderson, H. Field demonstration of coupling ion-exchange resin with electrochemical oxidation for enhanced treatment of per- and polyfluoroalkyl substances (PFAS) in groundwater. Chem. Eng. J. Adv. 2022, 9, 100216.
- Schaefer, C.E.; Choyke, S.; Ferguson, P.L.; Andaya, C.; Burant, A.; Maizel, A.; Strathmann, T.J.; Higgins, C.P. Electrochemical transformations of perfluoroalkyl acid (PFAA) precursors and pfaas in groundwater impacted with aqueous film forming foams. Environ. Sci. Technol. 2018, 52, 10689–10697.
- Schaefer, C.E.; Andaya, C.; Maizel, A.; Higgins, C.P. Assessing continued electrochemical treatment of groundwater impacted by aqueous film-forming foams. J. Environ. Eng. 2019, 145, 06019007.
- Zhuo, Q.; Deng, S.; Yang, B.; Huang, J.; Wang, B.; Zhang, T.; Yu, G. Degradation of perfluorinated compounds on a boron-doped diamond electrode. Electrochim. Acta 2012, 77, 17–22.
- Nzeribe, B.N.; Crimi, M.; Mededovic Thagard, S.; Holsen, T.M. Physico-chemical processes for the treatment of per- and polyfluoroalkyl substances (PFAS): A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915.
- Maldonado, V.Y.; Becker, M.F.; Nickelsen, M.G.; Witt, S.E. Laboratory and semi-pilot scale study on the electrochemical treatment of perfluoroalkyl acids from ion exchange still bottoms. Water 2021, 13, 2873.
- Soriano, A.; Schaefer, C.; Urtiaga, A. Enhanced treatment of perfluoroalkyl acids in groundwater by membrane separation and electrochemical oxidation. Chem. Eng. J. Adv. 2020, 4, 100042.
- Le, T.X.H.; Haflich, H.; Shah, A.D.; Chaplin, B.P. Energy-efficient electrochemical oxidation of perfluoroalkyl substances using a Ti4O7 reactive electrochemical membrane anode. Environ. Sci. Technol. Lett. 2019, 6, 504–510.
- Soriano, Á.; Gorri, D.; Urtiaga, A. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Res. 2017, 112, 147–156.
- Soriano, Á.; Gorri, D.; Urtiaga, A. Membrane preconcentration as an efficient tool to reduce the energy consumption of perfluorohexanoic acid electrochemical treatment. Sep. Purif. Technol. 2019, 208, 160–168.
- Soriano, Á.; Gorri, D.; Biegler, L.T.; Urtiaga, A. An optimization model for the treatment of perfluorocarboxylic acids considering membrane preconcentration and BDD electrooxidation. Water Res. 2019, 164, 114954.
- Sunka, P.; Babický, V.; Clupek, M.; Lukes, P.; Simek, M.; Schmidt, J.; Cernák, M. Generation of chemically active species by electrical discharges in water. Plasma Sources Sci. Technol. 1999, 8, 258–265.
- Thagard, S.M.; Takashima, K.; Mizuno, A. Chemistry of the positive and negative electrical discharges formed in liquid water and above a gas–liquid surface. Plasma Chem. Plasma Process. 2009, 29, 455–473.
- Singh, R.K.; Multari, N.; Nau-Hix, C.; Anderson, R.H.; Richardson, S.D.; Holsen, T.M.; Mededovic Thagard, S. Rapid removal of poly- and perfluorinated compounds from investigation-derived waste (IDW) in a pilot-scale plasma reactor. Environ. Sci. Technol. 2019, 53, 11375–11382.
- Palma, D.; Richard, C.; Minella, M. State of the art and perspectives about non-thermal plasma applications for the removal of PFAS in water. Chem. Eng. J. Adv. 2022, 10, 100253.
- Jovicic, V.; Khan, M.J.; Zbogar-Rasic, A.; Fedorova, N.; Poser, A.; Swoboda, P.; Delgado, A. Degradation of low concentrated perfluorinated compounds (PFCs) from water samples using non-thermal atmospheric plasma (NTAP). Energies 2018, 11, 1290.
- Hayashi, R.; Obo, H.; Takeuchi, N.; Yasuoka, K. Decomposition of perfluorinated compounds in water by DC plasma within oxygen bubbles. Electr. Eng. Jpn. 2015, 190, 9–16.
- Yasuoka, K.; Sasaki, K.; Hayashi, R. An energy-efficient process for decomposing perfluorooctanoic and perfluorooctane sulfonic acids using DC plasmas generated within gas bubbles. Plasma Sources Sci. Technol. 2011, 20, 034009.
- Mededovic Thagard, S.; Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Bohl, D.G.; Paek, E.; Dickenson, E.R.V. Plasma-based water treatment: Development of a general mechanistic model to estimate the treatability of different types of contaminants. J. Phys. D Appl. Phys. 2017, 50, 014003.
- Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Dickenson, E.R.V.; Mededovic Thagard, S. Plasma-based water treatment: Efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ. Sci. Technol. 2017, 51, 1643–1648.
- Wang, Y.; Niu, J.; Zhang, Z.; Long, X. Sono-photocatalytic degradation of organic pollutants in water. Prog. Chem. 2008, 20, 1621–1627.
- Xia, C.; Lim, X.; Yang, H.; Goodson, B.M.; Liu, J. Degradation of per- and polyfluoroalkyl substances (PFAS) in wastewater effluents by photocatalysis for water reuse. J. Water Process Eng. 2022, 46, 102556.
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12.
- Zhu, Y.; Xu, T.; Zhao, D.; Li, F.; Liu, W.; Wang, B.; An, B. Adsorption and solid-phase photocatalytic degradation of perfluorooctane sulfonate in water using gallium-doped carbon-modified titanate nanotubes. Chem. Eng. J. 2021, 421, 129676.
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A.; Wu, M.; Xu, G. Photocatalytic removal of perfluoroalkyl substances from water and wastewater: Mechanism, kinetics and controlling factors. Chemosphere 2017, 189, 717–729.
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 2020, 185, 116219.
- Wang, M.; Cai, Y.; Zhou, B.; Yuan, R.; Chen, Z.; Chen, H. Science of the total environment removal of PFASs from water by carbon-based composite photocatalysis with adsorption and catalytic properties: A review. Sci. Total Environ. 2022, 836, 155652.
- Xu, T.; Zhu, Y.; Duan, J.; Xia, Y.; Tong, T.; Zhang, L.; Zhao, D. Enhanced photocatalytic degradation of perfluorooctanoic acid using carbon-modified bismuth phosphate composite: Effectiveness, material synergy and roles of carbon. Chem. Eng. J. 2020, 395, 124991.
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21.
- Rodriguez-Freire, L.; Balachandran, R.; Sierra-Alvarez, R.; Keswani, M. Effect of sound frequency and initial concentration on the sonochemical degradation of perfluorooctane sulfonate (PFOS). J. Hazard Mater. 2015, 300, 662–669.
- Fernandez, N.A.; Rodriguez-Freire, L.; Keswani, M.; Sierra-Alvarez, R. Effect of chemical structure on the sonochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environ. Sci. 2016, 2, 975–983.
- Kewalramani, J.A.; Wang, B.; Marsh, R.W.; Meegoda, J.N.; Rodriguez Freire, L. Coupled high and low-frequency ultrasound remediation of PFAS-contaminated soils. Ultrason. Sonochem. 2022, 88, 106063.
- Didenko, Y.T.; McNamara, W.B.; Suslick, K.S. Temperature of Multibubble Sonoluminescence in Water. J. Phys. Chem. A 1999, 103, 10783–10788.
- Ciawi, E.; Rae, J.; Ashokkumar, M.; Grieser, F. Determination of temperatures within acoustically generated bubbles in aqueous solutions at different ultrasound frequencies. J. Phys. Chem. B 2006, 110, 13656–13660.
- Hung, H.-M.; Hoffmann, M.R. Kinetics and mechanism of the sonolytic degradation of chlorinated hydrocarbons: Frequency effects. J. Phys. Chem. A 1999, 103, 2734–2739.
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Sci. Technol. 2005, 39, 3388–3392.
- Meegoda, J.; Kewalramani, J. Coupled High and Low-Frequency Ultrasound Systems and Methods for Remediation of Contaminated Solids. U.S. Patent Application No. 17/154,872, 29 July 2021.
- James Wood, R.; Sidnell, T.; Ross, I.; McDonough, J.; Lee, J.; Bussemaker, M.J. Ultrasonic degradation of perfluorooctane sulfonic acid (PFOS) correlated with sonochemical and sonoluminescence characterisation. Ultrason. Sonochem. 2020, 68, 105196.
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Kinetics and mechanism of the sonolytic conversion of the aqueous perfluorinated surfactants, perfluorooctanoate (PFOA), and perfluorooctane sulfonate (PFOS) into inorganic products. J. Phys. Chem. A 2008, 112, 4261–4270.
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Sonochemistry and sonoluminescence under dual-frequency ultrasound irradiation in the presence of water-soluble solutes. J. Phys. Chem. C 2008, 112, 10247–10250.
- Gole, V.L.; Fishgold, A.; Sierra-Alvarez, R.; Deymier, P.; Keswani, M. Treatment of perfluorooctane sulfonic acid (PFOS) using a large-scale sonochemical reactor. Sep. Purif. Technol. 2018, 194, 104–110.
- Pee, G.-Y.; Rathman, J.F.; Weavers, L.K. Effects of surface active properties on the cavitational degradation of surfactant contaminants. Ind. Eng. Chem. Res. 2004, 43, 5049–5056.
- Son, Y.; Lim, M.; Cui, M.; Khim, J. Estimation of sonochemical reactions under single and dual frequencies based on energy analysis. Jpn. J. Appl. Phys. 2010, 49, 07HE02.
- Cao, H.; Zhang, W.; Wang, C.; Liang, Y. Sonochemical degradation of poly- and perfluoroalkyl substances—A review. Ultrason. Sonochem. 2020, 69, 105245.
- Dükkancı, M.; Gündüz, G. Ultrasonic Degradation of Oxalic Acid in Aqueous Solutions. Ultrason. Sonochem. 2006, 13, 517–522.
- Kanthale, P.; Ashokkumar, M.; Grieser, F. Sonoluminescence, sonochemistry (H2O2 Yield) and bubble dynamics: Frequency and power effects. Ultrason. Sonochem. 2008, 15, 143–150.
- Supercritical Water Oxidation (SCWO)—Enviro Wiki. Available online: https://www.enviro.wiki/index.php?title=Supercritical_Water_Oxidation_(SCWO) (accessed on 22 May 2022).
- Schlosky, K.M. Supercritical phase transitions at very high pressure. J. Chem. Educ. 1989, 66, 989.
- Krause, M.J.; Thoma, E.; Sahle-Damesessie, E.; Crone, B.; Whitehill, A.; Shields, E.; Gullett, B. Supercritical water oxidation as an innovative technology for PFAS destruction. J. Environ. Eng. 2022, 148, 05021006.
- EPA. Air Pollution Control Technology Fact Sheet. Available online: https://www3.epa.gov/ttncatc1/dir1/fthermal.pdf (accessed on 15 September 2022).
- EPA. Per-and Polyfluoroalkyl Substances (PFAS): Incineration to Manage PFAS Waste Streams Background. US EPA Tech. Brief. 2020.
- Solo-Gabriele, H.M.; Jones, A.S.; Lindstrom, A.B.; Lang, J.R. Waste type, incineration, and aeration are associated with per- and polyfluoroalkyl levels in landfill leachates. Waste Manag. 2020, 107, 191–200.
- Wang, B.; Yao, Y.; Chen, H.; Chang, S.; Tian, Y.; Sun, H. Per- and polyfluoroalkyl substances and the contribution of unknown precursors and short-chain (C2–C3) perfluoroalkyl carboxylic acids at solid waste disposal facilities. Sci. Total Environ. 2020, 705, 135832.
- Winchell, L.J.; Ross, J.J.; Wells, M.J.M.; Fonoll, X.; Norton, J.W.; Bell, K.Y. Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: A state of the science review. Water Environ. Res. 2021, 93, 826–843.
- Hogue, C. Incineration May Spread, Not Break down PFAS. Available online: https://cen.acs.org/environment/persistent-pollutants/Incincerators-spread-break-down-PFAS/98/web/2020/04 (accessed on 18 September 2022).
- Stoiber, T.; Evans, S.; Naidenko, O.V. Disposal of products and materials containing per- and polyfluoroalkyl substances (PFAS): A cyclical problem. Chemosphere 2020, 260, 127659.
- Crunden, E.A. Defense Department Hits the Brakes on PFAS Incineration. Available online: https://www.eenews.net/articles/defense-department-hits-the-brakes-on-pfas-incineration/ (accessed on 18 September 2022).
- U.S.C. H.R.2591 PFAS Waste Incineration Ban Act of 2019. 2019. Available online: https://www.govinfo.gov/ (accessed on 9 September 2022).
- U.S.C. Public Law 116-92-National Defense Authorization Act for Fiscal Year 2020. 2019. Available online: https://www.govinfo.gov/ (accessed on 9 September 2022).
- Horst, J.; McDonough, J.; Ross, I.; Houtz, E. Understanding and managing the potential by-products of PFAS destruction. Groundw. Monit. Remediat. 2020, 40, 17–27.
- Stockenhuber, S.; Weber, N.; Dixon, L.; Lucas, J.; Grimison, C.; Bennett, M.; Stockenhuber, M.; Mackie, J.; Kennedy, E. Thermal Degradation of Perfluorooctanoic Acid (PFOA). In Proceedings of the 16th International Conference on Environmental Science and Technology, Rhodes, Greece, 4–7 September 2019.
- Altarawneh, M.; Almatarneh, M.H.; Dlugogorski, B.Z. Thermal decomposition of perfluorinated carboxylic acids: Kinetic model and theoretical requirements for PFAS incineration. Chemosphere 2022, 286, 131685.
- Sasi, P.C.; Alinezhad, A.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Xiao, F. Effect of granular activated carbon and other porous materials on thermal decomposition of per- and polyfluoroalkyl substances: Mechanisms and implications for water purification. Water Res. 2021, 200, 117271.
- Xiao, F.; Sasi, P.C.; Yao, B.; Kubátová, A.; Golovko, S.A.; Golovko, M.Y.; Soli, D. Thermal decomposition of PFAS: Response to comment on “thermal stability and decomposition of perfluoroalkyl substances on spent granular activated carbon”. Environ. Sci. Technol. Lett. 2021, 8, 364–365.
- Crownover, E.; Oberle, D.; Kluger, M.; Heron, G. Perfluoroalkyl and polyfluoroalkyl substances thermal desorption evaluation. Remediat. J. 2019, 29, 77–81.
- Yamada, T.; Taylor, P.H.; Buck, R.C.; Kaiser, M.A.; Giraud, R.J. Thermal degradation of fluorotelomer treated articles and related materials. Chemosphere 2005, 61, 974–984.
- Taylor, P.H.; Yamada, T.; Striebich, R.C.; Graham, J.L.; Giraud, R.J. Investigation of waste incineration of fluorotelomer-based polymers as a potential source of PFOA in the environment. Chemosphere 2014, 110, 17–22.
This entry is offline, you can click here to edit this entry!
