Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Zika virus (ZIKV) is a Flavivirus and was first isolated in a sentinel monkey kept for studying mosquito-borne diseases, and was also later isolated from Aedes africanus mosquitoes, confirming its vector-borne transmission, in the Ziika forest of Uganda.
- apoptosis
- placenta
- ER stress
1. Introduction
Zika virus (ZIKV) is a Flavivirus and was first isolated in a sentinel monkey kept for studying mosquito-borne diseases, and was also later isolated from Aedes africanus mosquitoes, confirming its vector-borne transmission, in the Ziika forest of Uganda [1]. Intracerebral inoculations of ZIKV in young mice showed extensive neurological lesions, while inoculation from the mice to non-human primates resulted in a self-limiting febrile condition in a few subjects. Later, neutralizing antibodies were found in both humans and monkeys on serological screenings [2]. The first human case was reported during the isolation process of the virus, wherein the clinical signs were described as pyrexia along with rashes by Simpson et al. in 1964 [3]. The occurrence of neurological abnormalities in infants born to pregnant mothers infected with ZIKV created concern regarding disease outbreak [4]. Currently, there is no Food and Drug Administration (FDA) approved vaccine or treatment for ZIKV infection.
2. ZIKV Epidemiology
The first widespread cluster of ZIKV outbreaks was reported from Yap Island in Micronesia [5]. Around 2013–2014, another outbreak with a considerable number of infections occurred in French Polynesia [6]. Reports of various modes of transmission other than mosquitoes and involvement of neurological disorders such as Guillain-Barre syndrome in a subset of the population were also observed during this outbreak [7,8]. The presence of vectors and travel-related introductions of ZIKV to a population without any prior exposure, along with other existing arboviral infections such as dengue and Chikungunya, may have favored the increased transmission of disease observed in the recent outbreaks [9,10,11,12]. In 2015 ZIKV had spread to Brazil, and later ZIKV spread to other parts of the American continent including Colombia, Honduras, Puerto Rico, the Dominican Republic, Jamaica, and Haiti [13]. In the mainland of the United States of America (USA), cases were also reported in the state of Florida in 2016 [14]
2.1. ZIKV Strains
Genetic changes in the ZIKV, involving complex interactions between the vector, human populations and non-human primate populations led to the evolution of the virus [15]. Two lineages of ZIKV are (1) Asian origin and (2) African origin. The African strain has two groups, the Ugandan versus the Nigerian group. The strain originally isolated from Rhesus macaque in the Ziika forest is MR-766, whereas IbH is the first strain isolated from the human blood in Nigeria [16]. The first isolated Asian ZIKV strain is from Malaysia with the prototype strain P6-740, and the cluster includes strains from Cambodia, French Polynesia and other Asian countries. In addition, some reports describe that the African strain is more cytotoxic to placental cells than Asian strains but both strains showed similar replicative efficiency [17].
ZIKV strains in the American continent that circulated from the 2015–2016 Brazil outbreak, evolved from the Asian lineage [18]. Travel-related to major sports events could have contributed to the spread of the virus from Pacific islands including French Polynesia to Brazil [19]. The presence of a new glycosylation motif in an asparagine residue at position 154 of envelope protein in the 2007 Yap strain- EC Yap and the French Polynesian H/PF/2013 strain could possibly explain the gain in their virulence when compared to MR766 which does not have this glycosylation motif [8].
2.2. Transmission of ZIKV
Usually, the disease is spread by the bite of the infected mosquito (Aedes aegypti, Aedes albopictus) [20]. The infection can also be vertically transmitted from infected mother to fetus. It can also be sexually transmitted, as ZIKV RNA is detected in semen samples of infected patients even after 6 months of infection [21,22], although only 3% of the total ZIKV cases account for sexually transmitted cases and a study suggests that semen suppresses the binding of ZIKV to cells [23]. Blood transfusions from infected individuals could also be a potential source of ZIKV infection early in the epidemic [24]. The virus replicates in the epithelial cells of the mosquitoes’ gut and later spreads to the mosquitoes’ salivary gland: then, the virus spreads to humans via a mosquito bite [25,26]. The receptors in the dermal fibroblasts, immature dendritic cells and keratinocytes facilitate viral entry and support viral replication [27]. Wild macaques are naturally susceptible to ZIKV infection [28]. The arbovirus infection follows a sylvatic cycle with non-human primates as the reservoir of the virus [29]. They serve as the connecting bridge for ZIKV circulation among mosquitoes and transmission to humans due to the extensive urbanization in the present-day scenario [30].
2.3. ZIKV Structure
ZIKV is icosahedral in symmetry, ~40 nm with a nucleocapsid ~25–30 nm and surface projections ~5–10 nm [31,32]. Its genome is 10.8 Kb with 5′ NCR (translation via a methylated nucleotide cap or a genome-linked protein) and 3′ NCR (translation, RNA packaging, cyclization, genome stabilization and recognition)[33,34,35]. The virion consists of an envelope (E protein) covering the majority of the surface with non-structural proteins NS1 for virion production, NS3, and NS5 are large, highly conserved proteins, NS2A, NS2B, NS4A, and NS4B are small, hydrophobic proteins and NS4B, NS5 are targets for evolution [35,36,37]. Functions of individual ZIKV proteins are enlisted in Table 1.
Table 1. Zika viral proteins and their function.
| Protein | Function |
|---|---|
| Envelope (E) | Host cell binding and membrane fusion [38] |
| Capsid | Viral protein surrounds nucleic acid [39] |
| Membrane protein | Proteolytic cleavage of a pre membrane protein from membrane protein in the Golgi apparatus results in the release of the virus [40] |
| NS1 | RNA replication [41] |
| NS2A | Modulates different components of the virus during assembly [42] |
| NS2B | Cofactor of NS3 protease [43] |
| NS3 | Protease and helicase domain for polyprotein possessing & nucleoside triphosphtase (NTPase)/RNA triphosphatase (RTPase) activities [43] |
| NS4A | Evasion of the innate immune response, associated with replication complex [44,45] |
| NS4B | Evasion of the innate immune response [46] |
| NS5 | Methyl transferase (MTase) and RNA dependent RNA polymerase (RdRp) [47] |
2.4. ZIKV Replication
Virus entry into the cell occurs by the initial recognition of host receptors by glycosylated regions on the envelope protein of the ZIKV [48]. Endocytosis of the infectious viral particle occurs by clathrin-coated vesicles. A low pH environment within the endosome facilitates conformational changes in the envelope protein of the virus, resulting in fusion to the endosome and thereby releasing the positive-strand RNA of the virus [47]. The positive strand becomes translated in the endoplasmic reticulum of the host cells into a polyprotein that is cleaved by the host cell proteases and the viral non-structural proteins such as NS3 and NS2B, which is a co-factor for protease. Non-structural proteins NS5 (RNA-dependent RNA polymerase) and NS3 (helicase) also replicate the positive-sense RNA strand to form a negative-sense RNA strand [49]. The negative-sense RNA strand serves as a template for further production of a new positive sense RNA strand. The newly produced positive sense RNA strand can either be translated or further used for viral genome replication [50]. After the assembly of structural proteins around the viral genome, they are translocated to the Golgi apparatus where they become mature virions by cleavage of the precursor membrane protein and exit the host cell [51] (Figure 1).
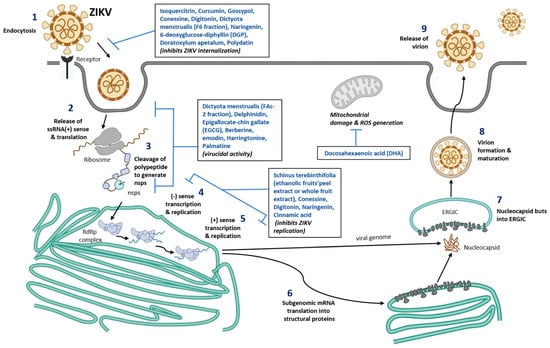
Figure 1. Schematic representation of nutraceuticals role in blocking ZIKV replication. Number 1–9 represent different stages of ZIKV infection, viral assembly, new viral formation, and release of mature virus from the infected cells. Nutrients compound identified that are known to inhibit various stages of viral infection are listed in the box inserts. ssRNA, single stranded ribonucleic acid; ERGIC, ER-Golgi intermediate compartment; RdRp, RNA dependent RNA polymerase; ROS, Reactive oxygen species; nsps, non-structural proteins of Zika virus.
2.5. Clinical Findings and Congenital Zika Syndrome
In normal healthy children and adults, ZIKV infection usually presents with a mild febrile disease with rashes and joint pain [52]. Pregnant women typically develop symptoms such as rashes during ZIKV infection [53]. ZIKV infection in pregnant women results in both congenital brain defects and ocular defects in the fetus. Brain defects include microcephaly, cerebral atrophy, subcortical calcifications, agyria, hydrocephalus and ventriculomegaly [54]. Ocular defects include microphthalmia, optic nerve defects, cataract, and intraocular calcifications. Congenital contractures, reduced musculoskeletal movements, dysphagia, hypertonia, hypotonia, seizures and irritability are also reported in infants with in utero ZIKV infection [55]. Further, a case-control study showed that women with ZIKV infection during the early stages of pregnancy were more likely to have babies with congenital Zika syndrome (CZS) [56]. ZIKV infection is also associated with the development of Guillain-Barre syndrome in some adults, which is an autoimmune condition affecting the nervous system [57].
2.6. Diagnosis, Treatment, and Prevention of ZIKV Infection
In suspected ZIKV cases, a diagnosis is usually based on laboratory confirmation using IgM detecting serological test or RT-PCR based on E and NS2B genes [58,59,60]. In a particular place when there are ongoing outbreaks, it is recommended for pregnant women to be tested for ZIKV infection [61]. Serology tests can detect ZIKV as early as one week after suspected infection, but cross-reacting antibodies from other Flaviviruses can result in false-positive serological results [62]. Measuring viral RNA copy number using RT-PCR can also be used to detect the initial viremia in urine samples, cord blood and placental samples at delivery [63].
Currently, there is no approved vaccine for the effective prevention of the disease [64]. Only supportive treatment is available if infected [65]. Implementing effective mosquito control strategies in places with ZIKV infection is crucial to break the chain of ongoing disease spread [66]. Avoiding travel to areas with ongoing ZIKV outbreaks, especially if pregnant or planning to become pregnant are some of the ways to reduce the risk of infection [67]. There us ab option of using genetically modified Aedes aegypti mosquitoes to reduce the population of wild type mosquitoes to control mosquito-borne disease, but it is considered an emerging risk [68]
2.7. ZIKV Vaccines and Drug Development
ZIKV vaccine development is challenged by the target audience; it must be safe for pregnant women and to prevent neurological disorders in adults and fetuses [69,70]. Despite the challenges, several vaccine candidates have entered preclinical animal studies and phase I clinical trials. Some of the vaccine candidates which have entered phase I clinical trials that are noteworthy to mention include DNA vaccines by Inovio Pharmaceuticals and NIH, whole purified inactivated vaccine by WRAIR/Sanofi Pasteur Limited and Live, Dengue virus vectored vaccine by Butantan Institute [69]. Another major issue in vaccine development and translation of the vaccine technology into use is that ZIKV outbreaks had waned, making it too challenging to test the effectiveness of the vaccine without ongoing active disease transmission, along with the slow decline in funding which supports vaccine development [71]. Several drug repurposing studies have been conducted and found to be effective against ZIKV infection. However, there are no FDA-approved drugs available for ZIKV infection because most of the drugs do not have enough data to support safety in pregnant women. Examples of existing drugs with anti-ZIKV activity are suramin, nitazoxanide, chloroquine (anti-protozoal drugs), niclosamide, ivermectin (anthelmintics), mycophenolic acid (an immunosuppressant drug), PHA-690509 (cyclin-dependent kinase inhibitor) and sofosbuvir (an anti-viral drug effective against hepatitis C virus, [72]. Sofosbuvir has shown promising results in preventing ZIKV transmission from mother to fetus in pregnant mice and pregnant non-human primate models [73,74]. Interestingly, for some phytochemical compounds a computation approach shows that Polydatin, Liquiritin, Cichoriin, Dihydrogenistin and Rhapontin shows high docking score compared to the Sofosbuvir. Especially, Polydatin has more capacity for receptor binding when compared to Sofosbuvir (Table 2). Thus, phytochemicals can be used as a cost-effective ZIKV inhibitors; however, biocompatibility and effectiveness have to be proved in non-computational research experiments [75].
This entry is adapted from the peer-reviewed paper 10.3390/nu15010124
This entry is offline, you can click here to edit this entry!
