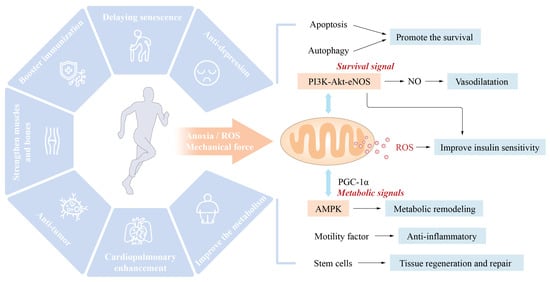
Mitochondria are vital organelles that mediate the body’s response to movement. The promotion of mitochondrial synthesis and enhancement of mitochondrial function through signalling molecules such as peroxisome proliferator-activated receptor-coactivator-1 (PCC-1) is essential for the health benefits of exercise [
3,
4]. According to Wang et al. [
5], cardiomyocyte apoptosis and vascular endothelial dysfunction might result in detrimental cardiovascular system remodeling, leading to the onset and progression of IHD. Exercise intervention primarily activated the PI3K/Akt pathway, raised the amount of anti-apoptotic factors, decreased pro-apoptotic factors, and greatly lowered cardiomyocyte apoptosis and unfavorable remodeling. By activating the PI3K/Akt/eNOS pathway, the level of NO can be increased, endothelial cell proliferation and migration can be stimulated, damaged endothelial cells can be repaired, the vascular endothelial function can be enhanced, the progression of IHD can be stabilized, and the disease state can even be reversed. Similarly, moderate aerobic exercise (16 m/min, 60 min/d, 5 days/wk) can activate the neuregulin-1 (NRG1)/epidermal growth factor receptor, ErbB, and fibroblast growth factor 21 (FGF21)/PI3K/AKT to inhibit myocardial apoptosis, reduce the MI area, reduce myocardial fibrosis, and promote cardiac repair and angiogenesis, and improve heart disease’s rational remodeling and cardiac function [
6]. Whereas, long-term and intensive aerobic activity may cause physiological hypertrophy of the heart and thereby enhance cardiac function. In contrast to pathological hypertrophy, physiological hypertrophy is not accompanied by pathological changes such as cardiac fibrosis. This type of exercise strongly triggers the IGF1-PI3K-Akt signalling pathway in the myocardium [
7] (
Figure 1).
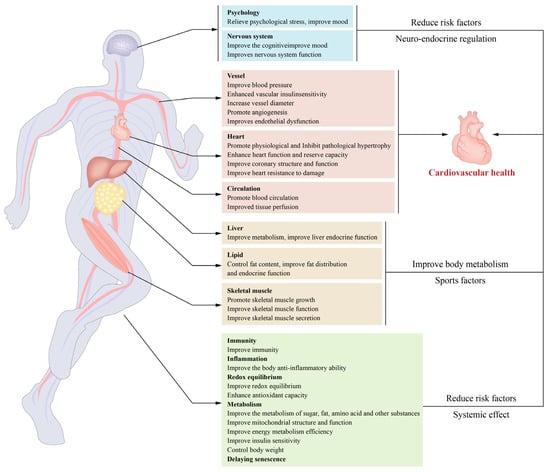
In addition, exercise may lower cardiovascular disease risk factors, modify the cardiovascular structure and function, and enhance insulin sensitivity and loss resistance, among other effects. Exercise-related molecule production by numerous tissues and organs contributes to metabolism and cardiovascular protection, highlighting the relevance of crosstalk between tissues and organs in the exercise-induced improvement of cardiovascular health.
2. Exercise Reduces the Risk Factors for Cardiovascular Disease and Its Main Mechanism
2.1. Exercise Improves the Body’s Metabolism
Metabolic syndrome, which is characterized by obesity, hyperlipidemia, impaired glucose tolerance, and insulin resistance, is a major risk factor for cardiovascular diseases, including hypertension, atherosclerosis, and coronary heart disease. Various forms of exercise may improve glucose, lipid, and insulin sensitivity [
8]. The molecular benefits include significantly lowered triglycerides, increased high-density lipoprotein levels, accelerated browning and thermogenesis in adipose tissue, enhanced fatty acid metabolism, increased muscle mass, and decreased body fat. A recent study has shown that the metabolic imbalance of amino acids is a major cause of cardiovascular and metabolic illness. Exercise may alleviate the metabolic problem of basal and branched-amino acids (BCAA) in pathological situations such as diabetes by increasing the cellular usage of amino acids and consequently enhancing cardiovascular, cardiometabolic, and metabolic health [
9,
10,
11,
12,
13].
2.2. Exercise Improves REDOX Balance and Chronic Inflammatory State
The underlying pathophysiological cause of numerous cardiovascular diseases is oxidative stress. Long-term exercise may improve the redox state of the body by boosting the antioxidant capacity, which is one of the most important processes through which exercise enhances cardiovascular health. During physical exercise, the contraction of skeletal muscle increases the generation of reactive oxygen species (ROS), which are primarily generated by the mitochondrial electron transfer pathway [
14,
15,
16]. ROS might cause cellular oxidative stress and are a type of active hazardous material that causes biological macromolecular damage. The production of adequate ROS is physiologically necessary. ROS generated during exercise may stimulate mitochondrial synthesis, activate insulin signalling, stimulate protein synthesis and muscle development, control gene expression, and boost the body’s antioxidant capacity. Long-term exercise may improve mitochondrial function, the body’s antioxidant capacity, and the maintenance of its oxidative level [
17]. Suppressing ROS generation during exercise reduces the health benefits of exercise [
18]. Exercise may also improve mitochondrial dynamics by suppressing excessive mitochondrial division in cardiovascular disorders (ischaemic heart disease, heart failure) [
19]. Acute exercise may have a similar “ischaemic preconditioning” effect on the heart by increasing the cardiac mitochondrial formation of ROS. Long-term physical activity may improve the mitochondrial antioxidant capacity and heart REDOX status [
20,
21]. Both aerobic exercise and resistance exercise substantially influence the homeostasis and function of mitochondria and are essential to the benefits of exercise on cardiovascular health.
Numerous chronic diseases, particularly metabolic cardiovascular disorders, throw the body into a state of low-grade chronic inflammation, which results in chronic non-communicable diseases (NCDs) and represents a significant cause of chronic diseases and accelerated ageing. Long-term aerobic exercise was reported to decrease chronic inflammation and is an effective technique for interrupting this vicious cycle. Similarly, previous studies have shown that aerobic exercise may reduce the inflammatory marker high-sensitivity C-reactive protein (hS-CRP) by 40% in coronary heart disease patients [
22]. Additionally, exercise may boost the synthesis of interleukin-6 (IL-6) in human skeletal muscle and reduce the production of tumour necrosis factor-α (TNF-α), interleukin-1 (IL-1), and other proinflammatory molecules, and exercise thus serves as an anti-inflammatory input [
23]. Furthermore, exercise may enhance fat metabolism, reduce visceral fat, and activate the hypothalamic–pituitary–adrenal axis and sympathetic nervous system to generate cortisol and other anti-inflammatory cytokines [
24]. Long-term exercise has a systemic influence on the redox balance and inflammatory state of the body and may enhance cardiovascular health directly or indirectly.
2.3. Exercise Slows Ageing
The ageing process is associated with structural and functional body changes and is a risk factor for chronic diseases such as cardiovascular disease. Long-term and consistent aerobic activity exerts an antiageing effect because it may encourage the migration of stem cells, resulting in a “youthful” body [
25]. Exercise, for example, decreases the activation of glial cells due to ageing and increases the glial cell volume by increasing the levels of insulin-like growth factor 1 (IGF1) and the vascular endothelial growth factor (VEGF). These growth factors boost the central nervous system’s insulin sensitivity and promote neurogenesis and angiogenesis. These activities may enhance cerebral blood flow, reduce amyloid deposition, and delay age-related neurodegenerative changes [
26]. Exercise may also enhance the ageing-related endocrine function of the hypothalamic–pituitary axis and the muscular and bone condition of elderly individuals [
27]. The effects of exercise on heart ageing include the following:
- ✓
-
Senile heart β adrenoceptor desensitization makes the heart less sensitive to adrenergic stimulation. Exercise can enhance the sensitivity of the myocardium to adrenergic stimulation and thus increases the cardiac functional reserve [
28].
- ✓
-
The heart loses its Ca
2+ processing capacity as it ages. The same stimulation reduces the intracellular Ca
2+ increase and the cell contractility. Exercise can enhance intracellular Ca
2+ processing capacity and cell contractility [
29].
Mitochondrial dysfunction is an important mechanism for modulating cardiac function, in which exercise improves mitochondrial dynamics and function to adjust the cardiac metabolism, reduce oxidative stress, and increase the cardiac reserve capacity [
30]. Moreover, vascular ageing is typically characterized by thickening and stiffening of large blood vessel walls, endothelial dysfunction, thrombosis-promoting changes in the vascular endothelium, proinflammatory and vasoconstrictive phenotype transformation, smooth muscle to secretory type changes, and reduced angiogenesis ability [
31]. Long-term aerobic exercise can ease and even partly reverse the vascular ageing alterations listed above [
32]. The fundamental mechanism through which exercise prevents ageing might be increasing the body’s antioxidant capacity and redox state because oxidative stress is a major cause of ageing.
2.4. Exercise Prevention and Treatment of Hypertension
Numerous scientific and clinical studies have shown that physical activity helps prevent and cure hypertension. Scientists have reported that all exercises (such as endurance, dynamic resistance, and isometric muscular strength training) reduce systolic blood pressure. Regular aerobic exercise may lower the resting blood pressure of hypertensive individuals by 57 mm Hg [
33]. Similarly, high-intensity interval training and continuous aerobic training have similar effects on lowering blood pressure. Still, high-intensity interval training has tremendous potential for enhancing cardiopulmonary endurance, vascular endothelial function, and insulin sensitivity [
34,
35]. It is currently believed that the mechanism through which exercise prevents and improves hypertension consists primarily of the following:
- ✓
-
Reducing the resting systolic.
- ✓
-
Improving the oxidative stress levels and regulating the renin–angiotensin system to affect vascular remodelling and blood vessels.
- ✓
-
Increasing insulin sensitivity and nitric oxide (NO) bioavailability and promoting vasodilation and tissue perfusion.
2.5. Muscle-Building Effects of Exercise and Cardiovascular Health
Skeletal muscle represents approximately 40% of the human body’s mass, which makes it the most conspicuous organ. In addition to carrying out the body’s motor function, skeletal muscle is one of the major organs involved in glucose metabolism and, by extension, glucose homeostasis. A decrease in skeletal muscle mass may impair glucose metabolism and tolerance. In addition to enhancing insulin sensitivity, the muscle-building effect of exercise promotes glucose metabolism and tolerance by enhancing blood glucose “pathways” (into the skeletal muscle) that serve to buffer increases in blood glucose. Moreover, muscle is an essential endocrine organ of the body that can regulate cardiovascular metabolism and function via the production of various exercise factors. Recently, it has shown an association between the amount of human muscle tissue and the incidence and mortality of cardiovascular disease [
36,
37]. Muscular atrophy (e.g., sarcopenia) is characterized by a decrease in muscle-derived carnitine, which is essential for fatty acid transport in the heart and skeletal muscle and thus leads to abnormal fatty acid metabolism. Sarcopenia is common in patients with heart failure due to the retention of the ejection fraction, which may result in cardiovascular dysfunction due to abnormal glucose and lipid metabolism and decreased production of exercise hormones [
38,
39].
2.6. Other
In recent years, many studies have investigated the mechanism through which the biological function of gut bacteria can influence cardiovascular health via their metabolites. Indeed, the time it takes for physical exercise to affect intestinal flora is linked to higher body fat, higher triglycerides, and better circulation of high-density lipoprotein cholesterol (HDL-c) and blood pressure. Intriguingly, the intestinal microbiota of top athletes and inactive individuals exhibit metagenomic and metabolomic differences, which shows that regular exercise might enhance metabolic health by improving the intestinal microbiota [
40,
41,
42]. For instance, Liu et al. reported that exercise may boost short-chain fatty acids and decrease branched-chain amino acid (BCAA) levels by enhancing the gut flora and thus improve glucose metabolism and insulin sensitivity [
43]. Also, mental and psychological variables contribute to the onset and progression of ischaemic heart disease. Exercise may alleviate psychological stress, lower the moderate=to-severe anxiety prevalence in coronary heart disease patients by 56% to 69%, and facilitate cardiac rehabilitation [
44]. In addition, physical activity may increase stem cell mobilization and tissue healing [
45]. These interactions are principal contributors to cardiovascular health (
Figure 2).
Figure 2. Exercise-induced cardiovascular benefits and underlying mechanisms: Exercise benefits cardiovascular health through its effects on the body. The primary mechanisms include enhancement of insulin sensitivity and metabolism in the cardiovascular system, reductions in oxidative stress and inflammation, the initiation of structural and functional remodelling in the cardiovascular system, the promotion of exerkine secretion from skeletal muscles and other tissues, and decreases in the risk factors for cardiovascular disease.
3. Exercise Improves the Cardiovascular Structure and Function
3.1. Remodeling of the Cardiovascular Structure and Function
Even moderate exercise reduces pathological ventricular hypertrophy, such as that caused by heart failure, and improves cardiac shape and function under pathological conditions. Exercise-induced vascular remodelling is characterized by increases in the arterial vessel diameter and arterial vessel wall thickness (including coronary arteries) [
46]. Likewise, exercise increases collagen and elastin content in atherosclerotic plaques while decreasing atherosclerosis-related adverse outcomes [
47]. This effect may be one of the mechanisms through which physical activity protects vital organ function and retards ageing [
48].
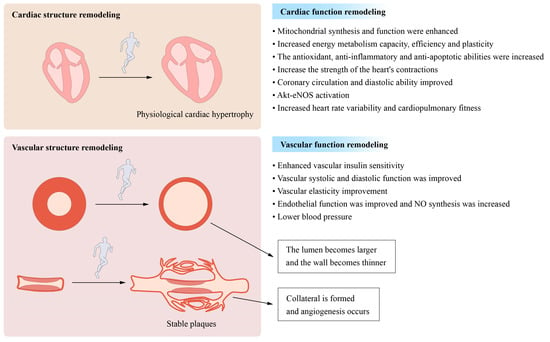
Exercise impacts blood vessels and increases the development of new blood vessels. Exercise, may increase the capillary formation and promote angiogenesis, while it may widen the arterial lumen through a process known as arteriogenesis (
Figure 3). This process may occur in existing blood vessels (including coronary arteries) and thus demonstrates substantial vascular system adaptability. A key regulator of angiogenesis is the vascular endothelial growth factor (VEGF), whose expression in skeletal and cardiac muscle may be increased by exercise [
49,
50].
Figure 3. Long-term moderate- and high-intensity exercise induces cardiovascular remodelling: Moderate- and high-intensity exercise performed over a prolonged period both causes and promotes structural and functional remodelling in the cardiovascular system. The structural remodeling process involves physiological cardiac hypertrophy, a decrease in the vascular wall thickness, and increases in the luminal width of conduit arteries. The process of functional remodelling is characterized by increases in cardiac contraction and dilatation and reductions in the heart rate and blood pressure.
Conversely, in atherosclerosis, exercise may enhance the amount of circulating endostatin and angiogenesis in plaque tissue, thus preventing the progression of atherosclerotic plaque, which may have important implications for the prevention and treatment of coronary heart disease [
51]. However, certain circumstances are required for angiogenesis and arteriogenesis to maintain tissue perfusion. The disturbance of these systems is one of the pathogenic mechanisms underlying several diseases, such as coronary heart disease. Angiogenesis and arteriogenesis may boost coronary collateral circulation, reduce myocardial damage during myocardial infarction, and improve blood perfusion and tissue healing [
52]. Exercise may stimulate the ischaemic myocardium, and angiogenesis and arteriogenesis can increase coronary collateral circulation and improve blood vessel and tissue healing perfusion. Endothelial dysfunction is one of the pathogenic mechanisms contributing to the development and progression of certain cardiovascular diseases. Physical activity may improve vascular endothelial function through mechanisms independent of cholesterol, blood pressure, glucose tolerance, and body weight [
53].
The effect of exercise on cardiovascular health is partially attributable to its direct mechanical stimulation of the cardiovascular system. One of the primary variables of cardiovascular function is mechanical force. For example, exercise-induced acceleration of the tissue blood flow increases shear forces, stimulating nitric oxide generation by vascular endothelial cells, widening capillaries, and improving blood perfusion to tissues and organs [
54]. Similarly, shear stress-induced movement increases blood flow and can directly induce vascular endothelial cells to secrete a movement factor. The latter, which is absorbed by heart muscle cells from outside the blood circulation and plays a role in cardiac protection, promotes interactions between the heart and blood vessels to affect cardiovascular regulation and protection [
55]. In a recent population-controlled study, stretching was found to be more effective for lowering blood pressure than brisk walking. The process may be related to the enhancement of vascular stiffness by mechanical stretching stimulation [
56].
The mechanism through which mechanical force stimulates and garners a response in the cardiovascular system remains unknown. Piezo receptors on the cell membranes of vascular endothelial cells, smooth muscle cells, and other tissues may sense mechanical force stimulation and initiate cellular calcium influx, resulting in changes in cellular functional activities. These receptors regulate cardiovascular function in response to exercise and are linked to the initiation and progression of cardiovascular diseases such as atherosclerosis, heart failure, and hypertension [
57,
58].
3.2. Improvements in Myocardial Mitochondrial Function and Metabolism
Numerous studies have shown that increased mitochondrial activity is directly connected to the cardioprotective benefits of exercise. Mitochondria govern metabolic, redox, and cell fate processes. The myocardium is the most mitochondrially dense tissue in the body, accounts for approximately 40% of the volume of cardiomyocytes, and serves as the heart’s main energy source. During exercise, the energy demands of the heart increase substantially, leading to an increase in ATP generation. In addition, the heart has access to almost all metabolic substrates. Under normal conditions, the major metabolic substrate of the heart is lipoic acid (40–70%). During exercise, the myocardium increases its use of fatty acids and lactic acid while decreasing its glucose usage. Prolonged exercise increases myocardial glucose use, particularly the glycolysis level, which is related to the establishment and progression of cardiac physiological hypertrophy [
59,
60]. Under normal conditions, the major metabolic substrate of the heart is lipoic acid (40–70%). During exercise, the myocardium increases its use of fatty acids and lactic acid while decreasing its glucose usage. Prolonged exercise increases myocardial glucose use, especially the glycolysis level, which is related to the establishment and progression of cardiac physiological hypertrophy [
61,
62]. Long-term exercise may increase mitochondrial dynamics and function and thus increases the pace and efficiency of cardiac metabolism and the adaptability of the metabolic switch and strengthen myocardial damage resistance [
63]. Exercise increases the transcription factors PGC-1, PPAR, NRF2, and others that govern cell function and mitochondrial homeostasis [
64].
3.3. Other
In addition to the abovementioned mechanisms, exercise influences cardiovascular function. For example, prolonged hyperexcitation of sympathetic neurons has a negative impact on the structure and function of the cardiovascular system, which is one of the causes of several cardiovascular diseases [
65]. Long-term aerobic exercise may enhance cardiovascular autonomic nervous system function and homeostasis, increase parasympathetic nerve activity, decrease sympathetic nervous activity, increase heart rate variability (HRV), thereby exerting a cardioprotective effect. Cardiorespiratory fitness (CRF) reduction due to ageing or long-term inactivity is also connected to the onset and development of cardiovascular diseases. According to a meta-analysis, all-cause mortality and cardiovascular mortality are lowered by 13 and 15%, respectively, for each additional metabolic equivalent (MET) of cardiopulmonary fitness [
66,
67]. Aerobic and resistance exercise may improve both cardiorespiratory fitness and cardiovascular health.