Harnessing the human immune system as a foundation for therapeutic technologies capable of recognizing and killing tumor cells has been the central objective of anti-cancer immunotherapy. There has been an increasing interest in improving the effectiveness and accessibility of this technology to make it widely applicable for adoptive cell therapies (ACTs) such as chimeric antigen receptor T (CAR-T) cells, tumor infiltrating lymphocytes (TILs), dendritic cells (DCs), natural killer (NK) cells, and many other. Automated, scalable, cost-effective, and GMP-compliant bioreactors for production of ACTs are urgently needed. The existing and most advanced systems for ACT manufacturing, including cell culture bags, G-Rex flasks etc.
- adoptive cell immunotherapy
- CAR-T
- T cell
- bioreactor
1. Introduction
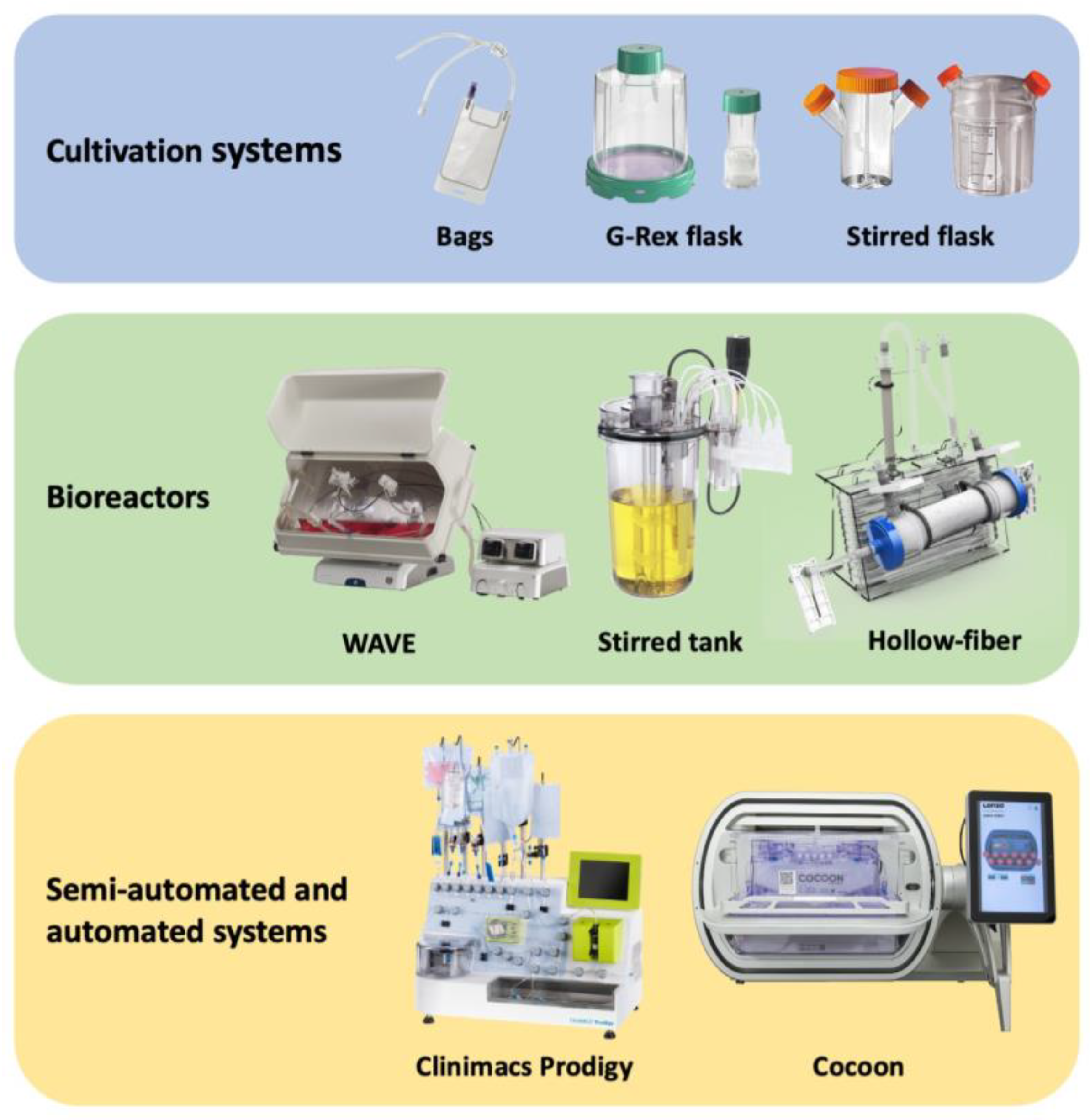
2. Cell Culture Bags
3. G-Rex Flask
| Culturing Method | Maximum Cell Density | Available Volume/ Size |
Advantages | Disadvantages |
|---|---|---|---|---|
| Stirred Flask | 1.7 × 106 cells/mL | 50 mL–35 L | Availability Scalability High level of aeration |
Controversial efficacy data for the cultivation of some cell products Careful selection of the shape of blades is required |
| G-Rex flask | 10–40 × 106 cells/cm2 | 2 cm2–500 cm2 8 mL–5 L |
High density of cultivation Relatively low cost Can be operated using standard laboratory equipment (laminar flow hood, cell culture incubator) |
Quality control check is required for each flask Additional steps needed for manual processing of the cell product Higher risk of contamination |
| Rocking motion bioreactors | 10 × 106 cells/mL | 250 mL–100 L | Scalable to large volumes Uniform cell mixing High level of media aeration Perfusion capability Sensors for pH, dO, temperature Aseptic media addition and sampling |
Low efficacy for some cell types Additional expensive equipment is required |
| Stirred tank bioreactors | 2 × 106 cells/mL | 250 mL–1 × 104 L | Great scalability Effective mixing with adjustable intensity High level of media aeration Range of sensors for monitoring cell culture Flexibility of control settings (rpm, shape of paddles, etc.) |
Controversial efficacy data for the cultivation of some cell products Additional expensive equipment is required Careful selection of the shape of blades is required |
| Hollow fiber | 1 × 109 cells/mL | area (cm2) to volume (mL) ratio = approx. 100–200 | Capability to culture cells at extremely high density Scalability Versatility (fibril configuration, cartridge size, etc.) Continuous addition of fresh medium and waste removal |
Additional expensive equipment is required Need to carefully select the membrane/fibril properties (porosity, packing density, diameter, etc.) Complicated collection of the cell product |
| CliniMACS Prodigy | 8 × 106 cells/mL | 500 mL | Semi-automated system Multicomponent device (all-in-one) Suitability for clinical application Availability of ready-made kits for various steps of the processes |
High cost of the system and consumables Cell expansion is carried out in a centrifuge chamber (CentriCult Unit, a part of CliniMACS Prodigy system) |
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering9120808
References
- Watanabe, N.; Mo, F.; McKenna, M.K. Impact of Manufacturing Procedures on CAR T Cell Functionality. Front. Immunol. 2022, 13, 876339.
- MacDonald, K.N.; Salim, K.; Levings, M.K. Manufacturing next-generation regulatory T-cell therapies. Curr. Opin. Biotechnol. 2022, 78, 102822.
- Zuliani, T.; David, J.; Bercegeay, S.; Pandolfino, M.-C.; Rodde-Astier, I.; Khammari, A.; Coissac, C.; Delorme, B.; Saïagh, S.; Dréno, B. Value of Large Scale Expansion of Tumor Infiltrating Lymphocytes in a Compartmentalised Gas-Permeable Bag: Interests for Adoptive Immunotherapy. J. Transl. Med. 2011, 9, 63.
- Li, M.; Chin, L.-Y.; Shukor, S.; Tamayo, A.; Maus, M.V.; Parekkadan, B. Closed Loop Bioreactor System for the Ex Vivo Expansion of Human T Cells. Cytotherapy 2019, 21, 76–82.
- Lapteva, N.; Vera, J.F. Optimization Manufacture of Virus- and Tumor-Specific T Cells. Stem. Cells Int. 2011, 2011, 434392.
- Somerville, R.P.; Devillier, L.; Parkhurst, M.R.; Rosenberg, S.A.; Dudley, M.E. Clinical Scale Rapid Expansion of Lymphocytes for Adoptive Cell Transfer Therapy in the WAVE® Bioreactor. J. Transl. Med. 2012, 10, 69.
- Bollard, C.M.; Gottschalk, S.; Huls, M.H.; Leen, A.M.; Gee, A.P.; Rooney, C.M. Good Manufacturing Practice-Grade Cytotoxic T Lymphocytes Specific for Latent Membrane Proteins (LMP)-1 and LMP2 for Patients with Epstein–Barr Virus-Associated Lymphoma. Cytotherapy 2011, 13, 518–522.
- Vera, J.; Brenner, M.; Dotti, G. Immunotherapy of Human Cancers Using Gene Modified T Lymphocytes. Curr. Gene Ther. 2009, 9, 396–408.
- Vera, J.F.; Brenner, L.J.; Gerdemann, U.; Ngo, M.C.; Sili, U.; Liu, H.; Wilson, J.; Dotti, G.; Heslop, H.E.; Leen, A.M.; et al. Accelerated Production of Antigen-Specific T Cells for Preclinical and Clinical Applications Using Gas-Permeable Rapid Expansion Cultureware (G-Rex). J. Immunother. 2010, 33, 305–315.
- Budiyati, A.D.; Pawitan, J.A. Integrating Bioreactor System to Speed Up Tumour Infiltrating Lymphocytes (TILS) Immunotherapy Commercialization. Int. J. Appl. Pharm. 2020, 12, 34–38.
- Bajgain, P.; Mucharla, R.; Wilson, J.; Welch, D.; Anurathapan, U.; Liang, B.; Lu, X.; Ripple, K.; Centanni, J.M.; Hall, C.; et al. Optimizing the Production of Suspension Cells Using the G-Rex “M” Series. Mol. Ther.-Methods Clin. Dev. 2014, 1, 14015.
- Xiao, L.; Chen, C.; Li, Z.; Zhu, S.; Tay, J.C.; Zhang, X.; Zha, S.; Zeng, J.; Tan, W.K.; Liu, X.; et al. Large-Scale Expansion of Vγ9Vδ2 T Cells with Engineered K562 Feeder Cells in G-Rex Vessels and Their Use as Chimeric Antigen Receptor–Modified Effector Cells. Cytotherapy 2018, 20, 420–435.
- Sarnaik, A.A.; Hamid, O.; Khushalani, N.I.; Lewis, K.D.; Medina, T.; Kluger, H.M.; Thomas, S.S.; Domingo-Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. J. Clin. Oncol. 2021, 39, 2656–2666.
- Gagliardi, C.; Khalil, M.; Foster, A.E. Streamlined production of genetically modified T cells with activation, transduction and expansion in closed-system G-Rex bioreactors. Cytotherapy 2019, 12, 1246–1257.
