More than 30,000 drugs have been developed for diverse diseases, of which 1100 drugs could potentially cause liver injury. In the United Kingdom, the incidence of drug-induced liver injury (DILI) was reported as 13.9 per 100,000 inhabitants [
1], while contemporary studies in China suggested a higher incidence of 23.8 per 100,000 persons with a different etiology from that of Western countries [
2]. As one of the most severe adverse drug reactions (ADRs), DILI can damage the liver, causing acute liver failure (ALF), and fulminant hepatic failure that eventually requires a liver transplant or causes death [
3,
4,
5]; however, due to the assorted clinical features and complex mechanisms of DILI, clinicians often fail to detect the condition early and miss the critical window to treat the patient effectively [
6]. Incidents of DILI have been the major reason for regulatory bodies to decline new drug applications, or for pharmaceutical companies to modify dosing and regimens, declare prescription warnings, or withdraw the drug entirely from the market [
7].
Currently, there are emerging preclinical human-relevant in vitro models used to evaluate the toxic injury of drug candidates to the liver. In these models, either single-cell type or multi-cell type assays can be performed [
8]. The main difference between these two kinds of assays is the number of cell types used in the experiments. Only one of the primary human hepatocytes, immortalized liver-derived cell lines (e.g., HepG2, HuH7) or hepatocyte-like cells derived from stem cells, are generally used in single cell-type in vitro models, while multiple cell lines or multicellular co-culture systems are used as the representative of in vivo cellular behavior in multicell type assays [
9]. Three-dimensional in vitro liver co-culture systems were also developed for the investigation of DILI, where cytochrome P450 (CYP450) inducibility and bile canaliculi-like structures are imitated [
10]. Besides cytotoxicity assays, other in vitro assays are conducted to research the specific mechanism of potential hepatotoxicity. For example, the glucose-galactose assay and oxygen uptake assay can be conducted for the determination of mitochondrial injury [
11]. Investigation for BSEP inhibition by drugs can be also a manner to evaluate DILI [
12]. In addition, covalent binding assays and reactive metabolite trapping are used to detect the formation of reactive metabolites that can commonly cause liver injury [
13].
Animal models also play an important role in the pharmacokinetics and toxicity researches of drug metabolism in vivo relevant to DILI. Several models of chimeric mice with humanized hepatocytes have been developed over the years, most of which require the damage of endogenous mouse hepatocytes followed by a transplant of human liver cells. Highly immunodeficient NOG mice (TK-NOG) are a humanized liver model expressing a herpes simplex virus type 1 thymidine kinase (HSVtk) transgene and mouse liver cells that were ablated after exposure to ganciclovir [
14]. Humanized liver Fah−/−/Rag2−/−/Il2rg−/− (FRG) mice were developed by Azuma et al. by transplanting human hepatocytes into FRG mice whose endogenous hepatocytes were damaged due to the genetic block of the tyrosine catabolic pathway [
15]. These humanized liver models exhibit comparable liver enzyme expression levels and activity to the donor livers [
16], as an alternative tool to study the potential damage to the human liver of drug candidates. In addition to mouse models with humanized livers, several human CYP-transgenic mouse models have been generated. CYP450 humanization in mice can be achieved through the cross-breeding of a human CYP-transgenic mouse with a mouse-CYP knockout mouse or directly knocking in the human genes to replace the mouse genes [
17]. However, CYP450 humanization in mice can only investigate the action of a single human CYP transgene on drugs, hence the limited significance of these models to human drug hepatotoxicity assessment [
18].
2. The Prediction of DILI by In Silico Models
2.1. Knowledge-Based Prediction
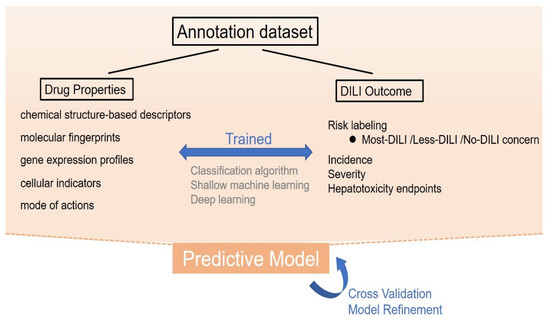
Computer algorithms can be established to predict DILI based on a series of training data, which can be applied clinically or during drug development. To facilitate analysis, most DILI events can be divided into the input cause and output result. Input causes include the properties of drugs, such as chemical structures, gene expression profiles, and cell and tissue images. These properties are used to determine the probability of DILI occurrence. Output results could include in vitro and in vivo hepatotoxicity, changes in biomarkers, or clinical adverse events related to DILI [
53]. By summarizing the rules between drug properties and DILI occurrence (see
Figure 2) using computer algorithms followed by proper and sufficient training, clinicians can predict the DILI risk of a new drug by preliminary properties.
Figure 2. The process of knowledge-based prediction. In knowledge-based prediction, drug properties including molecular descriptors, molecular fingerprints, gene expression profiles, cellular indicators, and their mode of action are used to develop a certain relationship rule with the existing drug-induced liver injury (DILI) outcome, using classification algorithm, shallow machine learning, or deep learning methods. Through sufficient training, validation, and refinements, these models can be applied to predict the DILI risk of a new drug by preliminary properties.
2.1.1. Cheminformatics-Based Model
Chemical structures are commonly associated with the bioactivity of drugs and closely relate to the occurrence and severity of DILI. As a result, the development of Quantitative Structure-Activity Relationship (QSAR) via in silico models plays an important role in the prediction and assessment of DILI. In QSAR, the structure of each chemical compound will be treated as a vector known as a molecular descriptor. Then the functional relationship between molecular descriptors and a DILI-related biological activity of molecules (represented by a scalar) will be constructed [
54]. Different chemical structure-based descriptors have been proposed, ranging from those with simple characteristics (that is molecular weight and number of carbon atoms) to sophisticated encodings typically referred to as “molecular fingerprints” [
55].
There are different computational programs to process the input information, which can be divided into explicitly coded decision rules and implicitly defined rules. Explicitly coded decision rules are commonly used when expert toxicology or hepatotoxicity knowledge is available, in which the assessment of hepatotoxicity is determined by a fixed classification algorithm based on chemical structures [
56]. However, most statistical models use implicitly defined rules, namely machine learning to achieve the same outcome. The primary algorithms typically can be trained with the ultimate decision rules obtained with optimization techniques [
57].
Expert Knowledge Approaches
With the expert knowledge approaches (explicitly coded decision rules), known drug information is used to identify specific fragments of molecules that are associated with DILI. These are generally called structural alerts [
58]. Egan et al. developed 74 computational alerts based on the structural and mechanistic information of 244 molecules using the Vertex cheminformatics platform (VERDI) that forms one of the published structural alert techniques. Of the 74 structural alerts, over 80 percent were related to the functional groups that are mainly converted to reactive toxic metabolites [
59]. Derek for Windows is another knowledge-based tool for predicting toxicity, covering carcinogenicity, mutagenicity, skin sensitization, hepatotoxicity, and reproductive toxicity [
60]. Greene et al. collected over 1266 chemicals and developed structure−activity relationships as structural alerts using Derek for Windows. The external evaluation of this model achieved an overall concordance of 56%, specificity of 73%, and sensitivity of 46% [
61].
Besides structural alerts, some classification algorithms are developed to utilize existing drug knowledge for the DILI judgment. For example, Zhu and Kruhlak proposed a scoring rule for post-marketing DILI data of 2029 drugs with 13,555 drug-adverse event pairs and classified them as DILI-positive or -negative according to their respective scores [
62].
2.1.2. Bioactivity-Based Model
To complement and improve chemical structure-based models, additional information in the form of biological drug properties such as gene expression data (i.e., genomic biomarkers for DILI prediction) and cellular indicators are necessary. Most gene expression data are accessible in some large-scale biomedical datasets such as the Connectivity Map (CMap) project, which is a collection of transcriptional expression data derived from cultured human cells treated with various compounds [
70] and Genomics-Assisted Toxicity Evaluation System (TG-GATEs), another large toxicogenomics database with higher diversity of data structures [
71]. The gene expression profile can be regarded as one of the drug features and correlated to hepatotoxicity outcomes for the prediction of DILI. Liu et al. collected different types of drug features, including chemical fingerprints, molecular descriptors, binding proteins, gene expression, therapeutic classifications, and different DILI endpoints such as liver failure, jaundice, biomarker increase, hepatomegaly, and hepatitis, and used these data to train logistic regression and random forest classifiers. The resultant areas under the receiver operating characteristic curve (AUC) were approximately 0.8 for certain DILI endpoints indicating that such a combination generally improved the model performance compared to only using a single feature [
72]. Li et al. developed an eight-layer Deep Neural Network (DNN) model for DILI prediction using transcriptomic profiles of human cell lines and the model also achieved a comparative AUC of 0.798 for the independent validation set [
73].
In addition, in vitro indicators determined by imaging assays can be a feature of chemical compounds, such as mitochondrial damage, oxidative stress, and intracellular glutathione. For example, Zhu et al. used human hepatocyte imaging assay technology (HIAT) descriptors that included several biochemical indicators (e.g., lipids and glutathione) of 156 DILI-positive and 136 DILI-negative compounds to build a DILI predictive model. Compared to the chemical structure-based model alone, the hybrid models combined with chemical structures and in vitro biological data could enhance the prediction accuracy of human hepatotoxicity [
74]. Puri et al. collected preclinical liver biopsy histopathology images for 10 common drugs that presented hepatic necrosis DILI phenotypes and input them into an artificial neural network to develop an AutoML model. This model was able to classify necrotic liver injury patterns accurately with an average precision of 98.6% [
75].
Mechanisms of drug action can also be considered during the modeling process. Wu et al. incorporated the mode of action of 333 drugs into the QSAR model, which was divided into active and inactive and yielded a predictive accuracy of 0.711 [
76].
2.2. Mechanism-Based Prediction
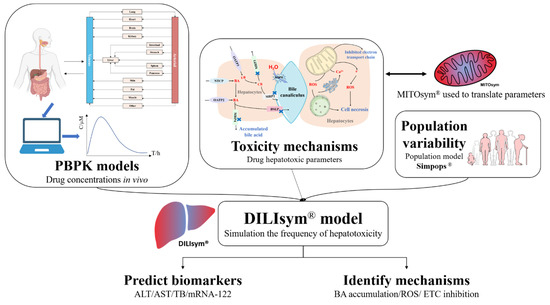
A mechanistic approach that is currently being developed—known as DILIsym—could provide a bottom-up prediction of liver safety liabilities in new drug candidates. It integrates pharmacokinetics exposure, mechanisms of hepatotoxicity, and interpatient variability into the modeling process to demonstrate the frequency and the extent of a new DILI in an average patient or a specific population [
77] (
Figure 3).
Figure 3. The illustration of DILIsym. DILIsym model integrates physiologically based pharmacokinetic (PBPK) model, hepatotoxic mechanisms of drugs, and population variability to simulate the occurrence and development of drug−induced liver injury (DILI), predicting the time−dependent release of biomarkers into serum and assisting the determination of DILI mechanisms. Mitochondrial dysfunction can be further investigated in MITOsym.
DILIsym can simulate the occurrent process of hepatotoxicity, incorporating submodels for the production of reactive metabolites, and generation of ROS (oxidative stress). Mitochondrial dysfunction can be further investigated in MITO-sym [
78], accumulation of toxic bile acids within the hepatocytes, lipotoxicity, as well as hepatocyte regeneration in response to injury [
79]. DILIsym can also analyze the interaction between hepatocytes and immune cells and simulate the production of innate immune responses in DILI [
80]. Combining the time-concentration profile in specific organs with the dose-effect relationship of each biological process in DILI production assessed with in vitro systems [
81], DILIsym will predict the time-dependent death of hepatocytes and hence the time-dependent release of biomarkers into serum [
82].
During the modeling process of DILIsym, a physiologically based pharmacokinetic (PBPK) model is created using available parameters related to the drug properties and physiological structures to estimate the time-dependent exposure of the drug in the region of interest. With the development of modeling theory, technology in the engineering field, and the popularization of computer technology and computing software, PBPK modeling techniques have matured considerably since their inception in the early 1930s [
83]. The PBPK model mathematically describes the physiological processes, including absorption, distribution, metabolism, and excretion (ADME) of chemicals within the body of an organism through computers [
84]. Unlike the classical atrioventricular model, most of the parameters of the PBPK model have physiological significance. Once the parameters are determined, the model can simulate and predict drug disposition in a specific organ or tissue under various conditions. As a result, the PBPK model is recognized as a “bottom-up” model [
85]. Due to its superior predictive capability, PBPK models have been applied widely in many fields, including the development of drug candidates the design of clinical trial protocols [
86], as well as the prediction of clinical drug-drug interactions [
87].
The first drug modeled by DILIsym was APAP where the model generated oxidative stress accounting for APAP overdosed hepatotoxicity. The modeling was used to propose the optimal treatment protocol with N-acetyl cysteine [
88]. Subsequently, Smith et al. predicted the clinical risk of hepatotoxicity of ubrogepant, telcagepant, and MK-3207 through DILIsym modeling. Telcagepant and MK-3207 were predicted to cause the rise out of the upper limit of normal ALT or total bilirubin at clinical pharmacologic doses, in accordance with clinical observation. Ubrogepant was predicted to be safe for the liver in all simulated individuals at all efficacious doses and a 10-fold higher amount than the proposed clinical dose, supporting the liver safety profile of ubrogepant in clinical trials [
89]. Diane et al. also used DILIsym to compare the potential liver toxicity of oral riluzole tablets versus BHV-0223, a novel sublingual formulation of riluzole. The results suggested that sublingual BHV-0223 had reduced hepatic exposure and, consequently, posed a lower risk of liver toxicity compared with riluzole oral tablets [
90].
Several fundamental DILI mechanisms are integrated into DILIsym, including mitochondrial toxicity, bile acid-mediated toxicity, and oxidative stress. By sequentially turning off each of these hepatotoxicity mechanisms and observing the DILI outcome change degree of each alteration, the predominant mechanism of hepatotoxicity can be found [
91]. By adjusting the parameters associated with hepatotoxicity mechanisms, the ultimate change in DILI outcome can be observed (i.e., parameter sensitivity analyses) to facilitate the conduct of in vitro assays. Therefore, DILIsym can be used to explore the profound effect on the human hepatotoxicity of specific drugs. Tolvaptan, an anti-hyponatremia drug for treating autosomal dominant polycystic kidney disease (ADPKD) is a good example to demonstrate such a feature by DILIsym. Tolvaptan had received a black box warning regarding hepatotoxicity. In clinical practice, ADPKD patients are assumed to be more susceptible to tolvaptan-induced liver injury based on the evidence that no signals of liver safety emerged during prior clinical trials or clinical use of tolvaptan in non-ADPKD patient populations. James et al. used DILIsym to simulate the impact of reduced biliary efflux, which was one of the common manifestations in ADPKD patients on tolvaptan-associated hepatotoxicity. They altered the biliary excretion parameters Vmax of tolvaptan and DM-4103, the main metabolite of tolvaptan, and observed the resultant changes in the pharmacokinetics of tolvaptan and DM-4103, bile acid, mitochondrial homeostasis, and clinical biomarker measures. The results showed that a reduction in the biliary excretion V
max of tolvaptan had a minor impact on tolvaptan pharmacokinetics and hepatotoxicity, but that of DM-4103 resulted in marked hepatic accumulation of DM-4103 and bile acids, reductions in hepatic ETC activity and ATP concentrations and increase in hepatotoxicity plasma biomarkers. This DILIsym model supported the hypothesis that impaired biliary efflux increased susceptibility to tolvaptan-associated hepatotoxicity observed in patients with ADPKD, and MRP2 played a more prominent role in tolvaptan-associated liver injury owing to the inhibition of DM-4103 on MRP2 [
92].