The prevalence of chronic, non-healing skin wounds in the general population, most notably diabetic foot ulcers, venous leg ulcers and pressure ulcers, is approximately 2% and is expected to increase, driven mostly by the aging population and the steady rise in obesity and diabetes. Non-healing wounds often become infected, increasing the risk of life-threatening complications, which poses a significant socioeconomic burden. Aiming at an improved management of infected wounds, a variety of wound dressings that release the antiseptic polyhexanide (poly(hexamethylene biguanide); PHMB), has been introduced in the wound-care market. An overview of the main characteristics and applications of PHMB and of the main fabrication methods and characteristics of commercial PHMB-releasing wound dressings is presented.
- poly(hexamethylene biguanide)
- polyhexamethylene biguanide
- polyhexanide
- PHMB
- membrane
- wound dressing
- drug release
- antimicrobial
- wound healing
1. Wound Dressings as Polymer Membranes
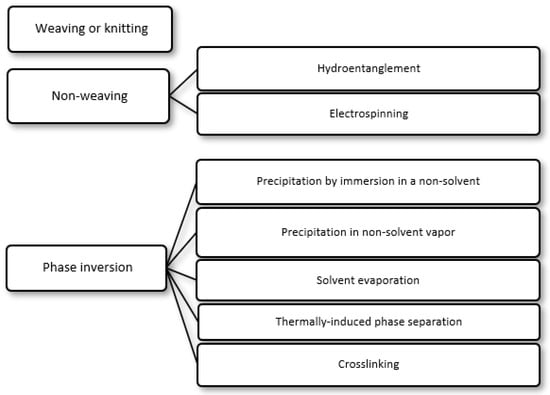
i) Precipitation by immersion in a non-solvent, in which precipitation occurs due to the exchange of the solvent in the polymer solution by a non-solvent miscible with the solvent present in a coagulation bath;
ii) Precipitation in non-solvent vapor, in which phase inversion occurs inside a closed chamber in which the non-solvent is in the vapor phase and causes precipitation of the polymer in the solution;
iii) Solvent evaporation (also known as solution casting), in which the solvent of a polymer solution cast on a substrate is allowed to evaporate or is removed by heating the polymer solution in an oven;
iv) Thermally induced phase separation, in which a solvent that dissolves the polymer at a given temperature loses this ability when the temperature is decreased, with the solvent being subsequently removed by extraction, evaporation or lyophilization (freeze-drying); and
v) Crosslinking, in which a polymer in solution is insolubilized by the formation of chemical bonds or by physical interactions between its molecular chains.
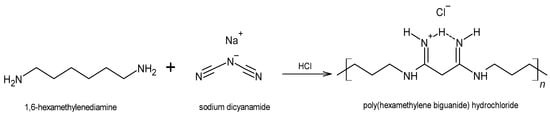
2. The Antiseptic Polyhexanide (PHMB)
When administered topically to wounds, PHMB is well tolerated, showing low cytotoxicity and poor skin penetration [9][25][34], and also promotes wound healing, by favoring tissue granulation on the wound surface [8]. In humans, repeated prolonged exposure to PHMB at concentrations of 2% may cause sensitization [35], most notably in patients with suspected allergic contact dermatitis [36]. However, this PHMB concentration is 4-times higher than the highest concentration employed for antisepsis. For instance, the wound irrigation/cleaning solution Serasept® (Serag-Wiessner GmbH & Co. KG, Naila, Germany) contains PHMB at a concentration of 0.03% or 0.04%, and Prontosan®, in all its three formats (solution, gel or spray; B. Braun Melsungen AG, Germany), contains 0.1% PHMB (in combination with a betaine antiseptic). In rats, acute skin toxicity was only observed for a concentration of 5% [25].
3. Loading of PHMB into Membranes
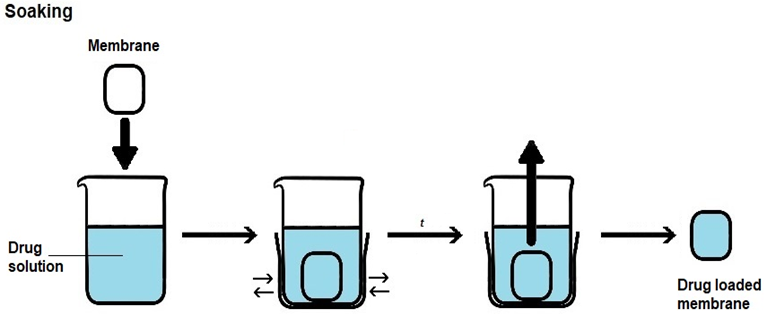
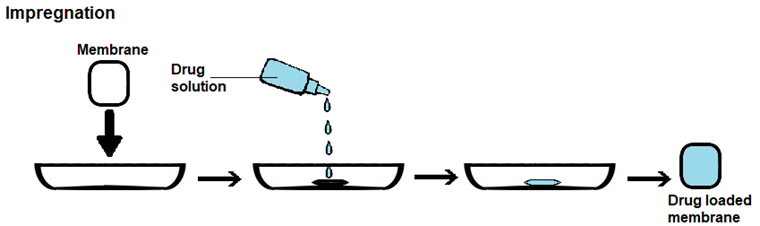
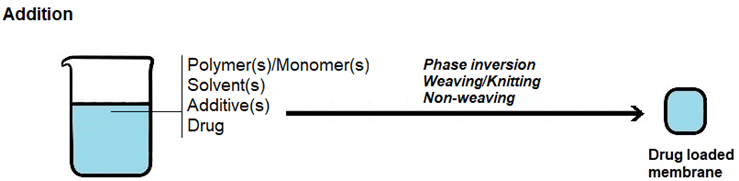
There are three main approaches to load a drug into a membrane with the aim of creating drug-releasing membranes, which are as follows (Figure 4):
i) Soaking, in which the membrane to be loaded is immersed in a drug solution for a certain period of time, usually under agitation;
ii) Impregnation, in which a drug solution is added to the membrane, being fully absorbed; and
iii) Addition, in which the drug is added to the formulation used to prepare the membrane.
Drug loading by soaking is the most straightforward method, but the loading yield can be low, as drug entry into the membrane occurs by diffusion across a concentration gradient. As such, some of the drug present in the solution will not be loaded. Loading yields depend not only on drug concentration, but also on other loading conditions, such as solvent, temperature, pH, ionic strength and time, as well as on drug/membrane interactions, molecular size of the drug, surface area, porosity and pore size of the membrane. In drug loading by impregnation, a contact between the membrane and a drug solution occurs but instead of occurrig by immersion of the membrane into the drug solution, the solution is added to the membrane until the membrane becomes fully saturated with drug solution. In drug loading by addition, the drug is added to the formulation employed to prepare the membrane. As such, it must be able to withstand the conditions employed in membrane preparation and, usually, it has to dissolve in the formulation. With this method, non-toxic materials and reagents have to be employed, since extensive drug loss would occur if the final drug-loaded membrane was washed to extract cytotoxic leachables. PHMB is amenable to all of these three membrane-loading approaches, due to its good hydrolytic, thermal and photo stabilities [46][47][52], solubility in water and ability to be dissolved in some organic solvents [9][53][54][55].
|
|
|
(a) |
|
|
|
(b) |
|
|
|
(c) |
Figure 4. Schematic representation of the methods employed in loading a drug into a membrane. (a) Drug loading by soaking. (b) Drug loading by impregnation. (c) Drug loading by addition.
4. Commercially Available PHMB-Releasing Wound Dressings (PRWDs)
| Commercial Name | Format | Material | PHMB Loading Mode/[PHMB] | Duration of Antimicrobial Activity | Sources |
|---|---|---|---|---|---|
| ActivHeal® PHMB | Foam, pad | Polyurethane | Impregnation/nd | 7 days | Advanced Medical Solutions, Ltd., Winsford, UK; [75] |
| CelluDress-PHMB | Pad | Polyester + viscose | nd 1 | nd | Medicareplus International Ltd., Wembley, UK; [58] |
| CurityTM AMD Antimicrobial Woven Sponges | Sponge, strip | Cotton | Impregnation/0.2% | Up to 3 days | Cardinal Health, Dublin, OH, USA; [76] |
| DracoFoam PHMB | Foam | Polyurethane | nd | Up to 7 days | Dr. Ausbüttel & Co. GmbH, Dortmund, Germany; [77] |
| ExcilonTM AMD | Sponge, gauze | Polyester + rayon | Impregnation/0.2% | Up to 3 days | Cardinal Health, Dublin, OH, USA; [76] |
| Fitostimoline® Plus Gauze | Gauze | nd | nd 2 | nd | Farmaceutici Damor S.p.A., Napoli, Italy |
| Gemcore360°TM PHMB Foam Border Dressing | Foam | nd | Impregnation/nd | Up to 7 days | GEMCO Medical, Hudson, OH, USA; [76] |
| Gemcore360°TM PHMB Non-Adhesive Foam Dressing | |||||
| Kendall™ AMD | Foam, disc | Polyurethane | Impregnation/0.5% | Up to 7 days | Cardinal Health, Dublin, OH, USA; [76] |
| Kerlix™ AMD | Gauze, sponge | Cotton | Impregnation/0.2% | Up to 3 days | |
| McKesson PHMB Hydrophilic Foam Dressing | Foam | Polyurethane | Impregnation/0.8–1% | Up to 7 days | McKesson Medical-Surgical, Irving, TX, USA; [76] |
| PuraPly® AM | Sheet, disc | Crosslinked ECM | nd | nd | Organogenesis Inc., Canton, MA, USA; [78] |
| Sterilux® AMD Antimicrobial Gauze | Gauze, sponge | Cotton | nd/0.1% PHMB (+0.02% BKC) | Up to 7 days | Hartmann USA, Inc., Rock Hill, SC, USA; [76] |
| Suprasorb® X + PHMB | Sheet | Bacterial cellulose | nd/0.3% | nd | Lohmann & Rauscher GmbH & Co. KG, Neuwied, Germany; [79] |
| Suprasorb® P + PHMB | Foam | Polyurethane | nd | ||
| Telfa™ AMD | Pad, island | Cotton | Impregnation/0.2% | Up to 3 days | Cardinal Health, Dublin, OH, USA; [76] |
| TielleTM PHMB | Foam | Polyurethane | Impregnation/nd | nd | 3M/KCI, St. Paul, MN, USA |
nd–Not disclosed by the manufacturer. 1 Contains PHMB complexed with an undisclosed surfactant. 2 Contains PHMB and an aqueous extract of Triticum vulgare. BKC–benzalkonium chloride; ECM–extracellular matrix.
This entry is adapted from the peer-reviewed paper 10.3390/membranes12121281
References
- Gupta, B.S.; Edwards, J.V. Textile materials and structures for topical management of wounds. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 55–104.
- Moore, G.K. Aspects of nonwoven bonding—The development and potential of hydroentanglement. TAPPI J. 1988, 71, 112–114.
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95.
- Ashjaran, A. Properties and applications of bacterial cellulose as a biological non-woven fabric. Asian J. Chem. 2013, 25, 783–788.
- Parikh, D.V.; Calamari, T.A.; Sawhney, A.P.S.; Sachinvala, N.D.; Goynes, W.R.; Hemstreet, J.M.; Von Hoven, T. Woven and nonwoven medical/surgical materials. Int. Nonwovens J. 1999, os-8, 1558925099OS-1558800117.
- Kesting, R.E. Phase inversion membranes. ACS Symp. Ser. 1985, 269, 131–164.
- Strathmann, H. Production of microporous media by phase inversion processes. ACS Symp. Ser. 1985, 269, 165–195.
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on wound antisepsis: Update 2018. Skin. Pharmacol. Physiol. 2018, 31, 28–58.
- Bernauer, U.; Bodin, L.; Celleno, L.; Chaudhry, Q.; Coenraads, P.-J.; Dusinska, M.; Duus-Johansen, J.; Ezendam, J.; Gaffet, E.; Galli, C.L.; et al. SCCS Opinion on Polyaminopropyl Biguanide (PHMB)—Submission III, SCCS/1581/16, Preliminary Version of 23 December 2016; Scientific Committee on Consumer Safety—European Commission: Luxembourg, 2016.
- Gao, Y.; Cranston, R. Recent advances in antimicrobial treatments of textiles. Text. Res. J. 2008, 78, 60–72.
- Magina, S.; Santos, M.D.; Ferra, J.; Cruz, P.; Portugal, I.; Evtuguin, D. High pressure laminates with antimicrobial properties. Materials 2016, 9, 100.
- Chivu, A.; Chindera, K.; Mendes, G.; An, A.; Davidson, B.; Good, L.; Song, W. Cellular gene delivery via poly(hexamethylene biguanide)/pDNA self-assembled nanoparticles. Eur. J. Pharm. Biopharm. 2021, 158, 62–71.
- Bromberg, L.; Raduyk, S.; Hatton, T.A. Functional magnetic nanoparticles for biodefense and biological threat monitoring and surveillance. Anal. Chem. 2009, 81, 5637–5645.
- Ren, L.; Chen, J.; Lu, Q.; Han, J.; Wu, H. Anti-biofouling nanofiltration membrane constructed by in-situ photo-grafting bactericidal and hydrophilic polymers. J. Membr. Sci. 2021, 617, 118658.
- Chen, Y.D.; Shu, C.; Duan, Z.H.; Xu, J.J.; Li, X.J.; Chen, F.; Luo, Q.J.; Li, X.D. Synthesis and characterization of an anti-caries and remineralizing fluorine-containing cationic polymer PHMB-F. Biomater. Sci. 2021, 9, 2009–2019.
- Wang, W.-Y.; Chiou, J.-C.; Chen, W.-X.; Yu, J.-L.; Kan, C.-W. A salt-free, zero-discharge and dyebath-recyclable circular coloration technology based on cationic polyelectrolyte complex for cotton fabric dyeing. Cellulose 2022, 29, 1249–1262.
- Britz, J.; Meyer, W.H.; Wegner, G. Poly(alkylene biguanides) as proton conductors for high-temperature pemfcs. Adv. Mater. 2010, 22, E72–E76.
- Tanış, E. New optoelectronic material based on biguanide for orange and yellow organic light emitting diode: A combined experimental and theoretical study. J. Mol. Liq. 2022, 358, 119161.
- Kazanskiy, N.L.; Butt, M.A.; Khonina, S.N. Carbon dioxide gas sensor based on polyhexamethylene biguanide polymer deposited on silicon nano-cylinders metasurface. Sensors 2021, 21, 378.
- Zhu, J.; Zhao, L.; Song, D.; Yu, J.; Liu, Q.; Liu, J.; Chen, R.; Sun, G.; Wang, J. Functionalized GO-doped double network antibacterial hydrogels for efficient uranium extraction from seawater. Desalination 2022, 540, 115993.
- Wang, Y.; Gu, M.; Ge, D.; Dong, Y.; Bai, L.; Han, Y.; Zhu, N. Polyhexamethylene biguanidine used as a new type sewage sludge conditioning agent: Effect on sludge dewaterability and mechanism. J. Environ. Manag. 2022, 315, 115146.
- Moore, K.; Gray, D. Using PHMB antimicrobial to prevent wound infection. Wounds 2007, 3, 96–102.
- Landelle, C.; von Dach, E.; Haustein, T.; Agostinho, A.; Renzi, G.; Renzoni, A.; Pittet, D.; Schrenzel, J.; François, P.; Harbarth, S. Randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy of polyhexanide for topical decolonization of MRSA carriers. J. Antimicrob. Chemother. 2015, 71, 531–538.
- Renzoni, A.; Von Dach, E.; Landelle, C.; Diene, S.M.; Manzano, C.; Gonzales, R.; Abdelhady, W.; Randall, C.P.; Bonetti, E.J.; Baud, D.; et al. Impact of exposure of methicillin-resistant Staphylococcus aureus to polyhexanide in vitro and in vivo. Antimicrob. Agents Chemother. 2017, 61, e00272-17.
- Hübner, N.O.; Kramer, A. Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin. Pharmacol. Physiol. 2010, 23 (Suppl. S1), 17–27.
- Larkin, D.F.; Kilvington, S.; Dart, J.K. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology 1992, 99, 185–191.
- Valluri, S.; Fleming, T.P.; Laycock, K.A.; Tarle, I.S.; Goldberg, M.A.; Garcia-Ferrer, F.J.; Essary, L.R.; Pepose, J.S. In vitro and in vivo effects of polyhexamethylene biguanide against Herpes simplex virus infection. Cornea 1997, 16, 556–559.
- Krebs, F.C.; Miller, S.R.; Ferguson, M.L.; Labib, M.; Rando, R.F.; Wigdahl, B. Polybiguanides, particularly polyethylene hexamethylene biguanide, have activity against human immunodeficiency virus type 1. Biomed. Pharmacother. 2005, 59, 438–445.
- Pinto, F.; Maillard, J.-Y.; Denyer, S.P.; McGeechan, P. Polyhexamethylene biguanide exposure leads to viral aggregation. J. Appl. Microbiol. 2010, 108, 1880–1888.
- Shoukat, K.; Pilling, S.; Rout, S.; Bradbury, J.; Humphreys, P.N. A systematic comparison of antimicrobial wound dressings using a planktonic cell and an immobilized cell model. J. Appl. Microbiol. 2015, 119, 1552–1560.
- Fabry, W.; Kock, H.J.; In-vitro activity of polyhexanide alone and in combination with antibiotics against Staphylococcus aureus.. J. Hosp. Infect. 2014, 86, 68-72, .
- Koburger, T.; Hübner, N.-O.; Braun, M.; Siebert, J.; Kramer, A.; Standardized comparison of antiseptic efficacy of triclosan, PVP-Iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. Antimicrob. Chemother. 2010, 65, 1712-1719, .
- Fabry, W.H.K.; Kock, H.J.; Vahlensieck, W.; Activity of the antiseptic polyhexanide against gram-negative bacteria. Drug. Resist. 2014, 20, 138-143, .
- Müller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287.
- European Chemicals Agency (ECHA). Opinion Proposing Harmonised Classification and Labelling at Community Level of Polyhexamethylene Biguanide or Poly(Hexamethylene) Biguanide Hydrochloride or PHMB; ECHA—Committee for Risk Assessment: Helsinki, Finland, 2011.
- Sukakul, T.; Dahlin, J.; Pontén, A.; Antelmi, A.; Bruze, M.; Hamnerius, N.; Hauksson, I.; Isaksson, M.; Lejding, T.; Svedman, C. Contact allergy to polyhexamethylene biguanide (polyaminopropyl biguanide). Contact Dermat. 2020, 84, 326–331.
- Kaehn, K. Polihexanide: A safe and highly effective biocide. Skin. Pharmacol. Physiol. 2010, 23, 7–16.
- O’Malley, L.P.; Hassan, K.Z.; Brittan, H.; Johnson, N.; Collins, A.N. Characterization of the biocide polyhexamethylene biguanide by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Appl. Polym. Sci. 2006, 102, 4928–4936.
- De Paula, G.F.; Netto, G.I.; Mattoso, L.H.C. Physical and chemical characterization of poly(hexamethylene biguanide) hydrochloride. Polymers 2011, 3, 928–941.
- Gray, D.; Barrett, S.; Battacharyya, M.; Butcher, M.; Enoch, S.; Fumerola, S.; Stephen-Haynes, J.; Edwards-Jones, V.; Leaper, D.; Strohal, R.; et al. PHMB and its potential contribution to wound management. Wounds 2010, 6, 40–46.
- Blackburn, R.S.; Harvey, A.; Kettle, L.L.; Payne, J.D.; Russell, S.J. Sorption of poly(hexamethylenebiguanide) on cellulose: Mechanism of binding and molecular recognition. Langmuir 2006, 22, 5636–5644.
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715.
- Ikeda, T.; Ledwith, A.; Bamford, C.H.; Hann, R.A. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim. Biophys. Acta Biomembr. 1984, 769, 57–66.
- Glukhov, E.; Stark, M.; Burrows, L.L.; Deber, C.M. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J. Biol. Chem. 2005, 280, 33960–33967.
- Chindera, K.; Mahato, M.; Sharma, A.K.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121.
- East, G.C.; McIntyre, J.E.; Shao, J. Polybiguanides: Synthesis and characterization of polybiguanides containing hexamethylene groups. Polymer 1997, 38, 3973–3984.
- Küsters, M.; Beyer, S.; Kutscher, S.; Schlesinger, H.; Gerhartz, M. Rapid, simple and stability-indicating determination of polyhexamethylene biguanide in liquid and gel-like dosage forms by liquid chromatography with diode-array detection. J. Pharm. Anal. 2013, 3, 408–414.
- Kramer, A.; Eberlein, T.; Müller, G.; Dissemond, J.; Assadian, O. Re-evaluation of polihexanide use in wound antisepsis in order to clarify ambiguities of two animal studies. J. Wound Care 2019, 28, 246–255.
- Zaki, A.M.; Troisi, A.; Carbone, P. Unexpected like-charge self-assembly of a biguanide-based antimicrobial polyelectrolyte. J. Chem. Phys. Lett. 2016, 7, 3730–3735.
- Wilson, K.S.; von Hippel, P.H. Transcription termination at intrinsic terminators: The role of the RNA hairpin. Proc. Natl. Acad. Sci. USA 1995, 92, 8793–8797.
- Mason, P.E.; Neilson, G.W.; Enderby, J.E.; Saboungi, M.-L.; Dempsey, C.E.; MacKerell, A.D.; Brady, J.W. The structure of aqueous guanidinium chloride solutions. J. Am. Chem. Soc. 2004, 126, 11462–11470.
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.C.; Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J. Mater. Sci. Mater. Med. 2015, 26, 245, .
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X.; Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. Appl. Biomater. 2012, 100, 1556-1565, .
- Llorens, E.; Calderon, S.; del Valle, L.J.; Puiggali, J.; Pollybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Mater. Sci. Eng. Mater. Biol. Appl. 2015, 50, 74–84, .
- Worsley, A.; Vassileva, K.; Tsui, J.; Song, W.; Good, L.; Polyhexamethylene biguanide:polyurethane blend nanofibrous membranes for wound infection control. Polymers 2019, 11, 915, .
- Brown, C.C. Antimicrobial Fabric for Surgical Drape. European Patent EP0136900b1, 28 September 1984.
- Barrett, S. Wound-bed preparation: A vital step in the healing process. Brit. J. Nurs. 2017, 26, S24–S31.
- Hagelstein, S.M.; Ivins, N. Treating recalcitrant venous leg ulcers using a PHMB impregnated dressing: A case study evaluation. Wounds 2013, 9, 84–90.
- Motta, G.J.; Trigilia, D. The effect of an antimicrobial drain sponge dressing on specific bacterial isolates at tracheostomy sites. Ostomy Wound Manag. 2005, 51, 60–62, 64–66.
- Kirker, K.R.; Fisher, S.T.; James, G.A.; McGhee, D.; Shah, C.B. Efficacy of polyhexamethylene biguanide-containing antimicrobial foam dressing against MRSA relative to standard foam dressing. Wounds 2009, 21, 229–233.
- Johnson, S.; Leak, K. Evaluating a dressing impregnated with polyhexamethylene biguanide. Wounds 2011, 7, 20–25.
- Sibbald, R.G.; Coutts, P.; Woo, K.Y. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing—Clinical trial results. Adv. Skin Wound Care 2011, 24, 78–84.
- Lee, W.R.; Tobias, K.M.; Bemis, D.A.; Rohrbach, B.W. In vitro efficacy of a polyhexamethylene biguanide-impregnated gauze dressing against bacteria found in veterinary patients. Vet. Surg. 2004, 33, 404–411.
- Shah, C. Polyhexamethylene biguanide (PHMB) treated wound dressings and vancomycin-resistant enterococci (VRE). Manag. Infect. Control 2007, 7, 26–34.
- Mueller, S.W.; Krebsbach, L.E. Impact of an antimicrobial-impregnated gauze dressing on surgical site infections including methicillin-resistant Staphylococcus aureus infections. Am. J. Infect. Control 2008, 36, 651–655.
- Cazzaniga, A.; Serralta, V.; Davis, S.; Orr, R.; Eaglstein, W.; Mertz, P.M. The effect of an antimicrobial gauze dressing impregnated with 0.2-percent polyhexamethylene biguanide as a barrier to prevent Pseudomonas aeruginosa wound invasion. Wounds 2002, 14, 169–176.
- Motta, G.J.; Milne, C.T.; Corbett, L.Q. Impact of antimicrobial gauze on bacterial colonies in wounds that require packing. Ostomy Wound Manag. 2004, 50, 48–62.
- Davis, S.; Mertz, P.M.; Cazzaniga, A.; Serralta, V.; Orr, R.; Eaglstein, W. The use of new antimicrobial gauze dressings: Effects on the rate of epithelialization of partial-thickness wounds. Wounds 2002, 14, 252–256.
- Wright, J.B.; Lam, K.; Olson, M.E.; Burrell, R.E. Is antimicrobial efficacy sufficient? A question concerning the benefits of new dressings. Wounds 2003, 15, 133–142.
- Fumarola, S.; Butcher, M.; Cooper, P.; Gray, D.; Russell, F.; Stringfellow, S.; Bertram, M.; Duguid, K.; Pirie, G.; Shand, S. A clinical audit of Suprasorb® X + PHMB. Wounds 2010, 6, 78–87.
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.C. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen. 2009, 17, 730–738.
- Piatkowski, A.; Drummer, N.; Andriessen, A.; Ulrich, D.; Pallua, N. Randomized controlled single center study comparing a polyhexanide containing bio-cellulose dressing with silver sulfadiazine cream in partial-thickness dermal burns. Burns 2011, 37, 800–804.
- Eberlein, T.; Haemmerle, G.; Signer, M.; Gruber Moesenbacher, U.; Traber, J.; Mittlboeck, M.; Abel, M.; Strohal, R. Comparison of PHMB-containing dressing and silver dressings in patients with critically colonised or locally infected wounds. J. Wound Care 2012, 21, 12–20.
- Muangman, P.; Nitimonton, S.; Aramwit, P. Comparative clinical study of Bactigras and Telfa AMD for skin graft donor-site dressing. Int. J. Mol. Sci. 2011, 12, 5031–5038.
- Forder, R.W.D. The management of a neuropathic diabetic foot ulcer using ActivHeal® PHMB foam. Diabet. Foot J. 2016, 19, 205–209.
- WoundSource. Woundsource: The Kestrel Wound Product Sourcebook. Available online: https://www.woundsource.com/ (accessed on 10 August 2021).
- Rembe, J.D.; Fromm-Dornieden, C.; Böhm, J.; Stuermer, E.K. Influence of human acute wound fluid on the antibacterial efficacy of different antiseptic polyurethane foam dressings: An in vitro analysis. Wound Repair Regen. 2018, 26, 27–35.
- Bain, M.A.; Thibodeaux, K.T.; Speyrer, M.S.; Carlson, E.; Koullias, G.J. Effect of native type I collagen with polyhexamethylene biguanide antimicrobial on wounds: Interim registry results. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2251.
- Kingsley, A.; Tadej, M.; Colbourn, A.; Kerr, A.; Aslan, C.B. Suprasorb® X + PHMB: Antimicrobial and hydrobalance action in a new wound dressing. Wounds 2009, 5, 72–77.