With the development of nanotechnology, various types of polymer-based drug delivery systems have been designed for biomedical applications. Polymer-based drug delivery systems with desirable biocompatibility can be efficiently delivered to tumor sites with passive or targeted effects and combined with other therapeutic and imaging agents for cancer theranostics. As an effective vehicle for drug and gene delivery, polyethyleneimine (PEI) has been extensively studied due to its rich surface amines and excellent water solubility.
1. Introduction
Cancer is a human disease characterized by abnormal cell proliferation and metastasis and poses a very significant threat to human health. Its occurrence is closely related to harmful environment, bad lifestyle, and heredity. Early diagnosis and treatment of cancer is the most important strategy to improve survival rates. Recently, nanotechnology has attracted extensive attention in the biomedical field, particularly in the early diagnosis and treatment of cancer [
1,
2].
The development of novel multifunctional nanoparticles (NPs) for cancer theranostics is one of the most important trends in the development of nanomedicine [
3,
4,
5,
6]. Compared with traditional drug delivery systems, NP-based drug delivery systems can not only improve the water solubility and stability of drugs, but also influence the distribution of drugs in vivo owing to the nanosized effects of NPs [
7]. In addition, the kinetics of drug release can be controlled through material design and surface modifications. More importantly, targeted molecules can be modified on the surface of NPs to specifically target tumor sites, thereby improving the bioavailability of drugs and reducing toxicity to off-target tissues [
8]. To date, various NPs have been developed for construction of NP-based drug delivery systems including liposomes [
9,
10], micelles [
11], nanogels [
12,
13], radionuclide-labeled NPs [
14,
15], and metal NPs [
16,
17,
18,
19].
Polyethyleneimine (PEI) is a cationic polymer molecule composed of abundant amine groups and two aliphatic carbons, and because of its specific structure and properties it has been widely used to stabilize or modify various inorganic hybrid NPs [
20]. As a cationic polyamine, PEI can interact with or bind to anionic residues of DNA templates and polymerase through electrostatic interaction, thus significantly improving their transfection efficiency [
21]. In addition, the strong positive surface potential of PEI presents obvious cytotoxicity to cells because of its abundant amine groups [
21]. Therefore, neutralizing the surface potential of PEI through various chemical or physical modifications can effectively reduce its cytotoxicity and improve biocompatibility. It is worth noting that these surface modifications not only improve the biocompatibility of PEI, but also enable it to acquire other functions, such as biomarker and targeting.
2. Overview of PEI
PEI is a commercially widely used cationic polymer containing primary, secondary, and tertiary amino groups in a ratio of 1:2:1 with strong positive charges [
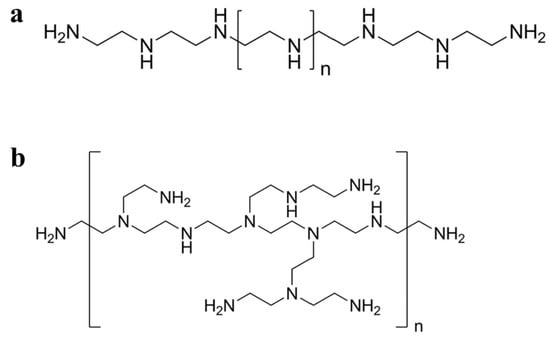
22]. PEI can be synthesized as linear PEI (
Figure 1a) or branched PEI (
Figure 1b) with a molecular weight ranging from 700 Da to 1000 kDa according to the degree of polymerization [
23]. PEI can be easily prepared using an AB-type monomer via a simple one-step reaction [
24]. In addition, PEI can be considered a low-cost option compared to dendrimers with the same molecular weight [
25]. PEI has been widely used in different fields because of its unique structure and abundant amino groups. For example, in industry, PEI can be used as a flocculant to remove oil present in synthetically produced water, or as a wet strength agent in paper-making and the manufacture of shampoo [
26,
27]. In biomedicine, PEI is widely used in enzyme immobilization [
28], virus immobilization on cellulose [
29], cell adhesion [
30], gene transfection [
31], and the synthesis of NPs to enhance their stability and anticancer efficacy [
32].
Figure 1. Schematic diagram of the chemical structures of (a) linear and (b) branched PEI.
Branched PEI is a hyperbranched polymer synthesized using the monomer method; that is, the cationic polymer is obtained by acid-catalyzed ring-opening polymerization of aziridine monomers [
33]. Each branch of secondary amines in the branched chain of hyperbranched PEI has 3–35 nitrogen atoms on average. This branch distribution can form a spherical internal structure, which can encapsulate NPs, drug molecules, and other small molecules. Furthermore, the lone pair electrons of nitrogen atoms in branched PEI can stabilize metal ions via coordination interaction. Therefore, branched PEI has a wide range of applications in gene transfection [
34,
35,
36], drug delivery [
37,
38], and molecular imaging [
25,
39].
Linear PEI contains only secondary amines, whereas branched PEI contains various types of amines, i.e., primary, secondary, and tertiary. Linear PEI is solid at room temperature, in contrast to branched PEI which is liquid at all molecular weights [
20]. Linear PEI is a high-charge cationic polymer and has been widely used in biomedical fields. For example, linear PEI has antibacterial properties against various pathogens and can therefore be used as a bacteriostatic agent [
40,
41]. Additionally, as a cationic polymer, linear PEI can form a polymer with nucleotides for gene transfer [
42]. Compared with branched PEI, linear PEI is an effective nonviral gene vector with higher cell viability and transfection efficiency [
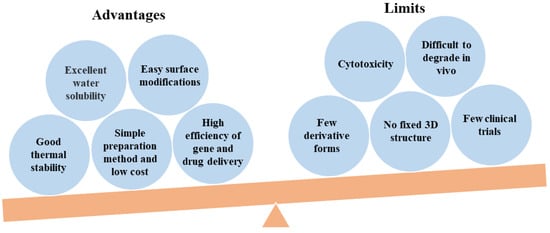
43]. A balanced picture of the PEI studies including advantages and limits has been presented in
Figure 2.
Figure 2. A balanced picture of PEI studies including advantages and limits.
3. PEI Modifications
As a cationic polymer, PEI contains abundant amino groups and as a result has a certain degree of cytotoxicity. Cationic PEI enters cells by adhering to negatively charged transmembrane heparanproteoglycans, which can cause cell damage through membrane destabilization [
44]. Additionally, the internalized PEI causes apoptotic cell death by forming pores in the mitochondrial membrane [
45,
46]. PEI is not well-degraded in organisms, and its cytotoxicity is closely related to its molecular weight and branching degree [
47]. Branched PEI with a higher molecular weight has a higher cytotoxicity. The surface amines of PEI can be shielded with simple modifications, thus significantly improving the biocompatibility of PEI [
21]. At present, the surface amines of PEI are mainly shielded with covalent bonds such as carboxylation, acetylation, and hydroxylation, or with electrostatic modification of negatively charged proteins. However, currently, there is a lack of systematic research to contrast the benefits and challenges of these approaches for the surface modifications of PEI. For example, Wen et al. improved the biocompatibility of PEI through carboxylation, acetylation, hydroxylation, and PEGylation [
21]. These methods effectively reduced or shielded the positive charge of the PEI, thus reducing cytotoxicity. Various functional groups including polyethylene glycol (PEG), folic acid (FA), hyaluronic acid (HA), fluorescent tags, and protein can be modified with PEI for biomedical applications [
24,
25,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58]. PEI modifications for biomedical applications in recent years are summarized in
Table 1.
Table 1. Summary of PEI modifications carried out in recent years.
|
Modification Types
|
Aims
|
Ref.
|
|
Carboxylation modification
|
Gene delivery, absorption of heavy metals in sewage.
|
[60,61,62,63]
|
|
Acetylation modification
|
Gene delivery efficiency improvement, cytotoxicity reduction.
|
[63,64,65]
|
|
Hydroxylation modification
|
Biocompatibility enhancement, gene delivery, transformation improvement of NPs.
|
[66,67,68]
|
|
PEG modification
|
Stability and transfection efficiency improvement.
|
[69,70,71]
|
|
FA modification
|
Tumor-targeted delivery.
|
[72,73]
|
|
HA modification
|
Tumor-targeted gene delivery, stability improvement.
|
[74,75]
|
|
Protein modification
|
Gene delivery, protein transduction.
|
[76,77,78]
|
|
FI modification
|
Fluorescence imaging.
|
[57]
|
4. Synthesis of PEI-Based NPs
NP-based drug delivery systems with high biostability, targeting, and biodegradation have significantly improved clinical efficacy [
97,
98,
99,
100]. Compared with traditional drug delivery systems, NP-based drug delivery systems can not only improve the water dispersibility and stability of drugs, but also significantly change the distribution and metabolism of drugs in vivo because of their specific size range (1–100 nm). In addition, the way of drug release can be controlled by appropriate design of delivery vehicles, drug molecule types, and loading modes, so as to achieve the best therapeutic effect. More importantly, in view of the specific receptor expression of cancer cells, NP-based drug delivery systems can be modified with targeted ligands to deliver drugs to specific tumor sites, thereby improving drug bioavailability and reducing toxicity to normal tissues.
PEI plays a crucial role in the construction of multifunctional NPs because of its unique structural features and abundant amino groups. Owing to the presence of hydrophobic cavities in hyperbranched PEI, small molecules, metal ions, and metal oxides can be effectively encapsulated to form stable NPs [
25,
55,
56,
58,
101,
102,
103,
104,
105]. For example, Sun et al. used PEGylated PEI to coat carbon nano-onion clusters (CNOCs) for cancer theranostics [
106]. The coating of PEGylated PEI can promote the phagocytosis efficiency of cells to the CNOCs. The CNOCs–PEI–PEG showed a cell uptake rate of 2.13 pg/cell, which was much higher than that of PBS and free CNOCs. The CNOCs–PEI–PEG was used as a photothermal and photoacoustic (PA) imaging agent for cancer theranostics because of its excellent photothermal conversion and cell phagocytosis efficiency.
In addition, the positively charged amino groups on the PEI surface can be bound to organic or inorganic anionic materials using electrostatic interaction. The lone electron of the amino group on the PEI surface can also coordinate with different metal atoms or metal ions to stabilize metal ions, metal oxides, or metal elements. For instance, Liu et al. conjugated PEI to the GO surface via amide bonds, which significantly improved the physiological stability and gene transfection rate of the GO [
107]. Sun et al. used linear PEI as a reducing and stabilizing agent to prepare AuNPs in a water bath at 60 °C, and studied the particle size changes of the AuNPs by regulating the feeding ratio of PEI and gold salt [
108]. Wang et al. systematically studied the preparation method of AuNPs based on hyperbranched PEI [
109]. Note et al. further studied the influencing factors of the size of AuNPs based on hyperbranched PEI, and found that AuNPs with a particle size of less than 10 nm could be obtained in either the aqueous or microemulsion phase when heated to 100 °C [
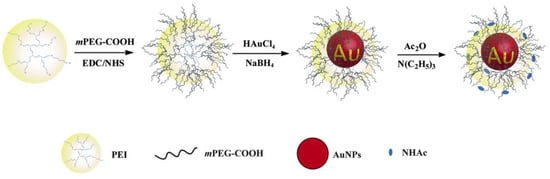
110]. The addition of strong reducing agents, such as sodium borohydride, resulted in preparation of AuNPs with diameters ranging from 2–5 nm. In the previous work, the researchers used PEGylated PEI to entrap and stabilize AuNPs (PP–AuNPs, as shown in
Figure 3) or metal ions (gadolinium and technetium ions) for in vivo CT or CT/MR (or SPECT/CT) dual-mode imaging of mice [
25,
55,
56,
58]. PEI also can stabilize iron oxide NPs and silver NPs for biomedical applications [
111,
112,
113,
114,
115,
116,
117,
118,
119].
Figure 3. Schematic illustration of the synthesis of PP–AuNPs.
5. PEI-Based Drug Delivery Systems
Since the FDA first approved liposomal amphotericin B as a delivery system in 1990, various delivery systems have been developed for the treatment of different diseases [
120]. The development of nanotechnology provides more options for the design of drug delivery systems.
As a cationic polymer, PEI can coat or conjugate drug agents, and numerous amino groups on its surface can be modified with various functional modifications [
121]. For example, targeting agents, such as FA [
122,
123], HA [
124,
125], lactic acid [
126,
127], transactivating protein [
128], and antibodies [
129,
130], modify PEI to target specific cancer cells; fluorescent labeling molecules, such as fluorescein isothiocyanate (FI), modify PEI for cell marking [
57]; and biocompatible agents, such as PEG and oligosaccharides, can improve the biocompatibility of PEI [
56,
131,
132,
133,
134]. The internal cavity of hyperbranched PEI and the large number of amino groups on the surface can be readily constructed as a nanoplatform which can effectively stabilize or entrap small biological molecules (e.g., DNA, siRNA, drugs) or metal ions. The unique physicochemical properties and low price of PEI promote its wide application in biomedicine.
5.1. PEI-Based Drug Delivery Systems for Cancer Treatment
PEI is a class of large-molecular-weight polymers, among which hyperbranched PEI has a hydrophobic cavity, dendritic three-dimensional structure, and plentiful positively charged amino groups on the surface, which provide the conditions for further chemical modifications [
21,
135,
136,
137]. PEI is an effective drug carrier for cancer treatments such as chemotherapy and gene therapy because of its unique structure, commercial availability, and low price.
Table 2 provides a detailed summary of PEI-based drug delivery systems for cancer therapy.
Table 2. PEI-based drug delivery system for cancer treatment.
|
Therapeutic Modalities
|
Therapeutic Agents
|
Cell Line Models
|
In Vivo Models
|
Ref.
|
|
Chemotherapy
|
DOX
|
HeLa
|
HeLa
|
[57]
|
|
MTX
|
HCT 116
|
/
|
[138]
|
|
PTX
|
HepG2
|
/
|
[139]
|
|
DOX
|
C6
|
/
|
[140]
|
|
DOX
|
HeLa
|
HeLa
|
[143]
|
|
DOX
|
4T1, HepG2
|
/
|
[149]
|
|
DOX
|
A549
|
/
|
[142]
|
|
DOX, siRNA
|
MDA-MB-231, HeLa, EAT
|
EAT
|
[150]
|
|
DOX
|
SKBR3
|
SKBR3
|
[151]
|
|
Gene therapy
|
pDNA
|
HeLa, 16HBE14o−, HepG2
|
/
|
[144]
|
|
pDNA
|
Huh7
|
Huh7
|
[145]
|
|
DNA
|
NIH/3T3
|
/
|
[45]
|
|
pDNA
|
HeLa
|
/
|
[51]
|
|
DNA
|
HeLa, CT26
|
CT26
|
[148]
|
|
mRNA
|
B16-OVA
|
B16-OVA
|
[152]
|
|
Other therapies
|
RNase A
|
MDA-MB-231
|
/
|
[153]
|
|
Oxidized mesoporous carbon nanospheres, pDNA
|
MCF-7
|
MCF-7
|
[154]
|
|
CAT-Ce6
|
T24
|
T24
|
[155]
|
|
GO, DTX, anti-miRNA21
|
MDA-MB-231
|
/
|
[156]
|
|
CuS, DTX, CpG
|
4T1
|
4T1
|
[157]
|
|
pDNA, 9B9 mAb
|
SMMC-7721
|
SMMC-7721
|
[158]
|
5.2. PEI-Based Drug Delivery System for Cancer Imaging
Contrast agents are widely used in molecular imaging to enhance imaging resolution. Owing to its unique physicochemical structure, PEI can effectively stabilize or encapsulate various agents for cancer imaging applications. A variety of imaging contrast agents can be constructed based on PEI including computed tomography (CT), magnetic resonance (MR), and single-photon emission CT (SPECT) imaging [
59,
165]. This section summarizes the progress of research concerning the use of PEI to construct multifunctional nanosystems as contrast agents for single-modal and multimodal molecular imaging.
Table 3 summarizes PEI-based imaging or imaging-guided cancer therapies.
Table 3. PEI-based imaging or imaging-guided cancer therapy.
|
Imaging Types
|
Imaging Agents
|
Cell Line Models
|
In Vivo Models
|
Ref.
|
|
CT
|
AuNPs
|
A549
|
A549
|
[53]
|
|
AuNPs
|
MCF-7
|
MCF-7
|
[200]
|
|
AuNPs
|
HeLa
|
HeLa
|
[201,202]
|
|
Bi2Se3 NPs
|
A549, U14
|
U14
|
[203]
|
|
MR
|
Gd ions
|
KB
|
KB
|
[187]
|
|
Superparamagnetic iron oxide nanocrystals
|
MCF-7/Adr
|
/
|
[191]
|
|
Superparamagnetic iron oxide NPs
|
Chondrolyte cells
|
/
|
[204]
|
|
Ultrasmall iron oxide NPs
|
4T1
|
4T1
|
[104]
|
|
Gd(OH)(3)-doped Fe3O4 NPs
|
KB
|
/
|
[205]
|
|
Fe3O4 NPs
|
HepG2
|
HepG2
|
[206]
|
|
Fe3O4 NPs
|
U87MG, HeLa
|
U87MG, HeLa
|
[90]
|
|
SPECT
|
131I
|
4T1
|
4T1
|
[196]
|
|
99mTc
|
C6
|
C6
|
[207]
|
|
MR/CT
|
AuNPs, Gd2O3
|
HeLa
|
HeLa
|
[208]
|
|
Fe3O4@Au nanostars
|
HeLa
|
HeLa
|
[92]
|
|
Fe3O4@Au nanocomposites
|
KB
|
/
|
[49]
|
|
Au-Gd NPs
|
HeLa
|
HeLa
|
[54,209]
|
|
MR/PA
|
Gd/CuS
|
KB
|
KB
|
[210]
|
|
SPECT/CT
|
99mTc, AuNPs
|
HCC-LM3
|
HCC-LM3
|
[58]
|
|
99mTc, AuNPs
|
SKOV-3
|
/
|
[105]
|
|
AuNPs, 131I
|
C6
|
C6
|
[121]
|
|
MR/CT/PA
|
Fe3O4 NPs, Au nanostars
|
HeLa
|
HeLa
|
[211]
|
|
MR/SPECT/PA
|
19F,99mTc, ICG
|
HepG2
|
HepG2
|
[193]
|
|
CT/MR/upconversion luminescence
|
Yb3+- and Gd3+-doped UCNPs
|
A2780
|
A2780
|
[212]
|
5.3. PEI-Based Drug Delivery Systems for Cancer Theranostics
The development of nanotechnology provides new strategies with regard to the combination of therapeutic drugs and imaging agents for imaging-guided cancer therapy, namely cancer theranostics [
18,
213,
214]. As a highly cationic polymer, PEI has the advantages of low cost, easy surface functionalization, stable chemical properties, and high loading of small molecules and NPs, enabling it to be used to construct PEI-based drug delivery systems for cancer theranostics. As an example, Shi et al. used an inverse mini-emulsion method to prepare PEI-based hybrid nanogels for incorporation with ultrasmall iron oxide NPs and the anticancer drug DOX for T
1 MR imaging-guided chemotherapy of tumors [
104]. The nanogels displayed excellent water solubility and colloidal stability, high DOX loading efficiency (51.4%), and a pH-dependent release of the DOX with an accelerated release rate under acidic pH. Compared to free ultrasmall iron oxide NPs, the nanogels showed a much higher r
1 relaxivity at 2.29 mM
−1 s
−1. Additionally, under the guidance of T
1-weighted MR imaging, the nanogels effectively inhibited tumor growth. HA-modified PEI-stable Fe
3O
4@Au core–shell nanostars (NSs) were used for trimodal CT-, MR-, and photothermal- imaging-guided PTT of tumors [
92]. Here, HA-modified PEI provided the NSs with desirable colloidal stability, biocompatibility, and targeted specificity to cancer cells overexpressing CD44 receptors. With the Fe
3O
4 core NPs and Au star shell, the NSs could be used as a contrast agent for efficient MR and CT imaging of tumors in vivo. Furthermore, because of the NIR absorption property, the NSs could also be used as a nanoprobe for thermal imaging and PTT of tumors.
Laponite (LAP) is a synthetic biodegradable nanoclay with a large specific surface area and cation exchange capacity [
215]. Combining LAP with PEI not only can improve the drug loading rate of the complex, but also produce good stability. Zhuang and colleagues created PEI-modified LAP using a polylactic acid-PEG-COOH spacer. The PEI-LAP was used as a nanoplatform to embed AuNPs and load DOX for targeted CT imaging and chemotherapy of tumors [
201]. The formed nanocomplexes displayed excellent colloidal stability and a high drug loading efficiency of up to 91.0 ± 1.8%, which significantly inhibited the growth of tumors and reduced the side effects of DOX. Alkoxyphenyl acylsulfonamide (APAS) as a zwitterionic polymer can enhance the cellular uptake of NPs at the pH of tumor microenvironment [
216]. Zhu et al. used APAS-linked PEI as a vehicle to entrap AuNPs and labeled it with radioactive
131I to enhance dual-modal SPECT/CT imaging-guided radiotherapy of tumors [
121]. Because of the charge conversion property of APAS, the AuNPs can change from neutral to positively charged in a weak acid environment, thus promoting cellular uptake. In addition, after
131I labeling, the therapeutic agents can enhance SPECT/CT dual mode imaging and radiotherapy of tumors in vivo.
This entry is adapted from the peer-reviewed paper 10.3390/jfb14010012