Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
Due to their commercial availability, superior processability, and biocompatibility, polymers are frequently used to build three-dimensional (3D) porous scaffolds. The main issues limiting the widespread clinical use of monophasic polymer scaffolds in the bone healing process are their inadequate mechanical strength and inappropriate biodegradation. Due to their mechanical strength and biocompatibility, metal-based scaffolds have been used for various bone regenerative applications.
- Tissue engineering
- Polymer-Mg composite
- Biomedical application
1. Introduction
Biopolymers made from biological sources and with adjustable biodegradability are gaining popularity. Depending on where they came from, biopolymers are divided into natural and synthetic classes [1]. Biopolymers convey their payloads to human bodies, which undergo regulated degradation to form nontoxic byproducts [2]. This distinct characteristic makes tissue engineering viable for treating patients with damaged or missing organs and tissues [3]. Three-dimensional structures for repairing soft and hard tissues are typically created using natural and synthetic biopolymers. However, the poor mechanical properties and the slow degradation rate of biopolymer limit the use of biopolymer in tissue engineering [4].
The search for newer biodegradable and biocompatible materials for tissue engineering has recently increased due to the challenges driven mainly by secondary surgeries associated with the issues of the existing metal implants. Accidents, injuries, and the natural aging process cause bone degeneration, which requires an implant to restore its function [5,6]. According to a 2019 Australian Orthopaedic Association (AOA) report, the need for hip, knee, and shoulder surgical procedures has exponentially increased, reaching a total number of 459,265 hip, 658,596 knee, and 40,130 shoulder surgeries in 2018 [7]. Similarly, bone fracture costs only around USD 32 billion annually in the US. Over 3 million bone surgeries are conducted worldwide every year [8]. That is the main reason for the importance of highly efficient and cost-effective bone fracture treatment. The orthopedic implant costs USD 45.9 billion in 2017 and is expected to reach USD 66.6 billion by 2025, showing a compound annual growth of 4.7% [9]. The current demand for temporary implants is also soaring for scaffolds, cardiac stimulators, and cardiovascular stents [10,11,12,13,14,15]. Such a steep increase in orthopedic implants has necessitated the acceleration in the research and development for finding new materials and fabrication methods to satisfy the medical requirements of biodegradability, biocompatibility, and on-demand personalized design and manufacturing at a low cost.
Implants, acting as a replacement for natural bones, should imitate the bone in its physical and biological properties as much as possible, that is, strength, hardness, chemical properties, degradation rate, biocompatibility in the physiological environment, and so on [16,17]. There are mainly two types of implants: (1) permanent implants, which are required to perform the function of the bone for life with no immature failure and additional replacement needs, and (2) temporary implants, which are required to fulfill the functions of a bone until the tissues are healed [18,19]. Temporary implants stay inside the body, fixed to the bones, until the body part is fully recovered, and then are removed [20,21,22]. Currently, commercially available implants are mostly made of metals and metallic alloys, including titanium, stainless steel, and Co-Cr alloys, due to their high mechanical strength, biocompatibility, and good corrosion resistance properties [23,24,25,26,27,28,29,30,31]. However, due to the differences between the elastic modulus of these implant materials and natural bones, they impart a stress shielding effect [32,33,34,35]. Another problem with these materials is the requirement of a second surgery to remove these implants after the healing of the fracture, causing pain to the patient. Such a process not only adds pain, discomfort, and risks to the patient but also costs about 30% of healthcare expenditures [36,37]. Studies have found that failing to remove these implants can cause severe allergic problems due to ion accumulation, causing osteolysis [36,37].
Therefore, using biodegradable materials that the body may dissolve is significant for these implants. Such biodegradable implants will replace the natural bone until its recovery without any complications. Owing to its low density (1.8 g/cm3), high strength-to-weight ratio, compressive yield strength, and comparable elastic modulus to natural bone, magnesium (Mg) and its alloys (with some bioactive materials) can be a perfect choice over the existing metal implants [38,39,40]. Mg, a crucial component of natural bone formation and the body’s fourth-most principal cation, has good biocompatibility and is among the ideal materials for temporary implants. On average, a healthy adult weighing 70 kg is known to have 21 to 35 g of stored magnesium [41,42]. Twenty percent of the available amount of Mg is kept in bones, followed by 35–40% in tissues and ligaments and 1% in bodily fluid [43]. In addition, over 300 enzyme-related processes rely on the availability of Mg, either directly or indirectly [44,45]. Mg has the second-highest daily permissible intake (420 mg) of all the nutritionally necessary substances [43]. Additionally, the byproducts of the breakdown of magnesium and its alloys can be safely taken by macrophages and eliminated through the urine without endangering physiological function [44].
Despite having several valuable qualities, the unpredictable deterioration rate of Mg-based implants in the physiological environment prevents their commercialization as temporary implants. Mg-based implants deteriorate significantly more quickly in the body fluid than the conventional aqueous solution due to high chloride ion concentration (96–106 mEq/L) and a pH of roughly 7.4–7.6 [46]. Additionally, the accelerated rate of gas bubble creation harms the patient’s health. The implants become particularly susceptible to unexpected failure and lose the necessary mechanical integrity to support the load during this deterioration phase. As a result, creating Mg-based implants with a controlled degradation rate to prolong the bone-required repair duration (between 24 and 32 weeks) is essential.
Tissue engineering scaffolds frequently use polymeric materials, such as polylactic acid (PLA), poly(lactide-co-glycolic acid) (PLGA), and polycaprolactone (PCL), due to their simple processing by 3D printing [47,48], biocompatibility, osteoinductivity, and negligible inflammatory response. Despite such appealing properties of the polymers that could be utilized as a 3D porous scaffold, inadequate mechanical strength and an improper degradation rate call for additional study in this area [49]. Metal particles as a reinforcement to a polymer matrix is an effective strategy for improving polymers’ biological behavior and mechanical properties. Due to its magnificent properties in biomedical applications, Mg can be an excellent addition to polymer materials, further enhancing biodegradable polymers’ mechanical and biological properties.
Polymer/Mg composite scaffolds must be designed with high dimensional accuracy and reproducible. Furthermore, these scaffolds should be designed according to the patient-specific anatomic requirements. 3D printing technology, as one of the advanced manufacturing techniques, offers high dimensional accuracy and excellent reproducibility with low-cost manufacturing. It can produce customized or personalized structures based on patient-specific anatomic data. Three-dimensional printing controls porosity and surface topology, determining the final implant’s mechanical, chemical, and biodegradation properties. Three-dimensional printing is known to be an easy, fast, and on-site manufacturing process, which can fabricate implants closely resembling native bone properties.
2. Suitability of Polymer/Mg Composites for Biomedical Applications
Tissue scaffolds for temporary implants must fulfill several essential functions. Magnesium, in combination with some polymers, meets many of these properties. The required properties undoubtedly change depending on the intended use and requirement. However, while we primarily concentrate on transient in orthopedic fixtures, biocompatibility, good mechanical properties, natural degradability, and osteogenesis are the most desired qualities.
2.1. Biocompatibility
Biocompatibility and nontoxicity are the two most crucial requirements for every implant material. Soon after a foreign object is inserted into a human body, interactions between the body tissues and implanted material start to happen. These reactions determine whether the body will accept the implant [50]. Biocompatibility for permanent implants depends on how well the newly formed tissue fuses with the implant surface. Contrastingly, temporary implants are intended to stabilize broken bone while it heals before degrading naturally in the body over time [8,51]. It is pretty concerning whenever a physiological component starts by negatively impacting the breakdown product. Consequently, nontoxic materials should be used for temporary implants.
The 3D porous bone scaffolds’ cellular activity is significantly affected by adding Mg particles into polymer matrices, such as PLA, PCL, and PLGA, during 3D printing. The presence of Mg is advantageous for cell growth, proliferation, and differentiation because composite scaffolds have more hydrophilicity, more bioactive area on their surface, and a moderately alkaline pH (pH 10), which is a favorable microenvironment for cell growth [52]. The amount of Mg in the polymer is a crucial element that needs to be tuned since an increase in the pH beyond 10 (pH > 10) harms cellular activity. Excess Mg (greater than 10 wt% (8.9 vol%) in PLGA and five wt% in PCL) has been reported to hurt cytocompatibility [53,54]. Figure 1 shows suitable cell attachment and proliferation on PCL/Mg composite scaffolds created by the FDM process. Cell viability (green = viable cells, red = dead cells) is markedly increased by adding five wt% Mg particles and decreased by further augmentation, as revealed in CLSM images (Figure 1a–f). Similarly, SEM photos demonstrate that cells in the PCL/Mg scaffold stretch, freely expand, and initiate filopodia, establishing a better cellular adherence to composite scaffolds. However, cells barely attached to the surface on the monophasic PCL scaffold with inappropriate conditions are unacceptable (Figure 1a–f) [53].
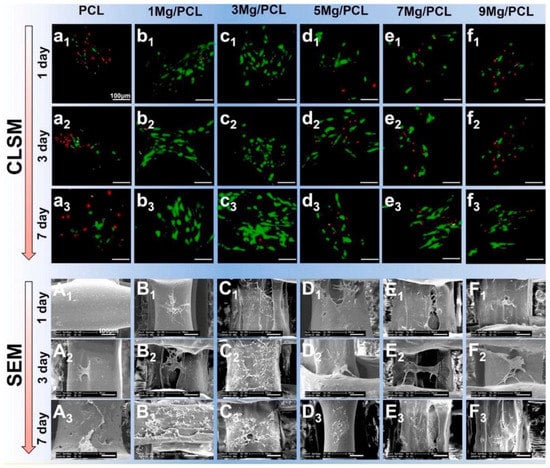
Figure 1. Cell adhesion and proliferation on 3D-printed PCL/Mg composite scaffolds (a–f) and SEM images of PLC/MG composite with different amounts of Mg (Mg = 0, 1, 3, 5, 7, and 9 wt %) (A–F) [53].
2.2. Mechanical Integrity
The material suitability to be employed as an orthopedic implant for a particular purpose is highly dependent on its mechanical qualities. Mechanical properties, such as elastic modulus, tensile strength, fatigue strength, hardness, and elongation, are among the rates given top priority. To prevent the “stress shielding effect”, the elastic modulus of biomedical implants should ideally have a similar value to the natural bone [32,33]. Biopolymers, owing to their excellent biocompatibility and biodegradability, have a strong possibility for scaffolds used in bone tissue engineering. However, they have poor mechanical qualities. This point of view claims that measures have been taken, such as customizing the pore structure and shape to overcome the substandard attribute qualities of the polymer 3D-printed scaffolds [55], modifying printing parameters [56], and incorporating other phases [57,58]. Among these efforts, the use of metallic powders as fillers is the most promising [59,60]. Similarly, adding Mg particles into the PLLA matrix demonstrated good mechanical properties (compressive strength and modulus of 5 wt% (3.6 vol%) Mg particles to the PLLA matrix considerably enhanced the compressive strength and modulus of 3D porous scaffolds by 114.5% and 85.7%, respectively). Adding more magnesium can negatively affect the mechanical characteristics of the porous composite scaffold made using the SLS process because of the particle agglomeration [61]. Alizadeh et al. reported a magnesium-reinforced PCL matrix [12]. The correct quantity of magnesium reinforcement strengthens mechanical capabilities, but too much magnesium weakens support due to the regional enhancement of magnesium microparticles in the PCL matrix. The compressive modulus of PCL/Mg scaffolds is summarized in Figure 2 [62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. Comparatively, PCL-based scaffolds have a lower compressive modulus than Mg-based scaffolds, which helps to limit the stress shielding effect. Additionally, Mg/PCL scaffolds created using 3DP had a greater compressive modulus than those made through electrospinning and salt leaching at a comparable porosity [63,64]. This scaffold’s mechanical attributes were like those of human cancellous bone, reducing the stress shielding effect. Ma et al. [77] observed an increase in compressive modulus by adding 20 wt% (18.1 vol%) Mg in the 3D porous PLGA scaffold. This enhancement was primarily ascribed to the PLGA/Mg composite scaffold’s increased load-bearing capability because Mg fillers have better strength and modulus.
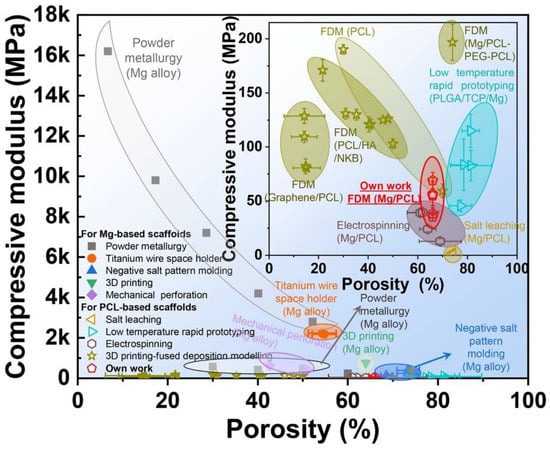
Figure 2. The compressive modulus of Mg-based and PCL-based scaffolds. Figure from [53].
2.3. Biodegradation
The relationship between the synthetic tissue scaffolds’ biodegradability or degradation rate and the rate at which bones mend is crucial. Because the hydrolytic breakdown pathway causes the polymeric chains to separate into monomers and water-solvable oligomers, biopolymers, including PLA, PCL, and PLGA, disintegrate in the physiological environment [49]. The total breakdown of the mentioned polymers in the body would take several years, however [78]. One of the significant problems with biodegradable polymer-based scaffolds is their sluggish disintegration rate, which needs more research. On the other hand, Mg, a very suitable metal with good biocompatibility, has a very high deposition rate. Therefore, incorporating Mg particles into these polymers in a specific ratio can have a controlled degradation rate matching that of the natural bone growth rate and having a good match of the mechanical properties similar to the natural bone. The incorporation of metallic powders into polymers is an effective strategy among the various methods used to increase the degradation of polymers without sacrificing biocompatibility, such as copolymerization [79], blending [80], and surface modification [81]. This is due to the outstanding ability to increase the strength and degradation rate simultaneously [61].
2.4. Osteogenic and Angiogenic Characteristics
The process of osteogenesis involves creating new tissues to mend a broken bone. It is a crucial necessity for temporary implants since the damaged bone needs to heal with the help of newly formed tissues and cells before implant disintegration [82,83]. The osteogenic activity of the synthetic polymers used in the 3D printing of 3D porous scaffolds needs to be improved because they are nonbioactive [84]. A promising strategy in this area has been the fabrication of polymer/metal composite scaffolds and introducing metallic fillers. Due to its inherent ability to promote osteogenesis, Mg is highly preferable compared with other bioactive materials. This suggests that Mg positively affects the osteogenic differentiation of 3D composite porous scaffolds and bone mineralization. According to reports, adding Mg to monophasic PLGA [54] and PCL [53] bone scaffolds remarkably increases the ability of genes relevant to osteoblast development and mineralization to express. The enhanced expression of neuronal calcitonin gene-related polypeptide (CGRP) by an Mg filler is suggested to be the cause of the polymer/Mg composite scaffolds’ robust bone formation.
Jing Bai et al. [53] have shown the general research methodology for Mg/PCL scaffolds for bone repair, as shown in Figure 3. The homogeneous distribution of Mg particles in PCL scaffolds is made possible by combining mixing, blending, and 3D printing. Scaffolds made of a Mg/PCL composite show good overall qualities and in vitro and in vivo responses. The Mg particles slowly degrade with a local pH increase in in-vitro degradation studies.
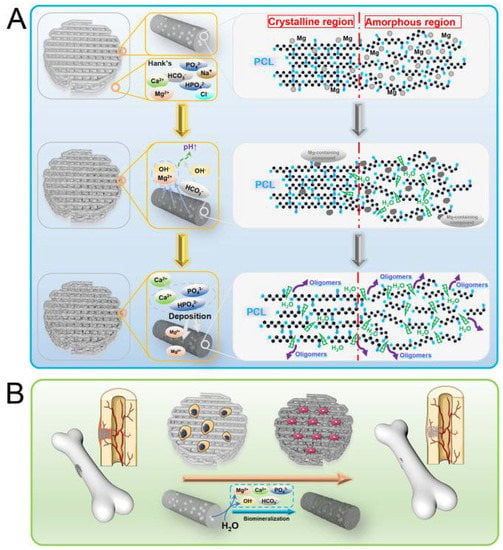
Figure 3. Reactions of Mg/PCL scaffolds for bone healing in vitro and in vivo, showing (A) degradation behavior and (B) biological activity [53].
Mg/PCL scaffolds encourage cell growth, proliferation, vascularization, and bone formation in in vitro biological investigation.
3. Conventional Techniques for Biopolymer and Polymer/Mg Composites
The characteristics of the resulting scaffolds are determined by the production method and material choices. Even though various techniques have been tried to make the scaffolds more porous and prevent cellular ingrowth, none of them have successfully created scaffolds thick enough to qualify as 3D scaffolds [92]. The numerous traditional methods used to create porous magnesium polymer composite scaffolds for biomedical purposes are briefly described in the following section.
Self-assembly: Self-assembly is the ability to assemble components into patterns and structures that can be used to create designs that mimic the ECM for bone tissue creation [93]. A trigger from the outside, such as a change in pH or temperature, might start the assembling mechanism in motion [94,95]. To create HA/Coll biohybrid composites, an approach inspired by biological mineralization was used [96]. The magnesium doping of the apatite phase nucleating on collagen caused the final composite (MgHA/Coll) to exhibit the physicochemical, structural, and morphological features typical of a newly formed natural bone. Intrinsic control mechanisms, including chemistry, morphology, and spatial distribution of the mineral phase, occur throughout the artificial mineralization process, much like they do during the natural biomineralization process. Despite its many benefits, this method produces scaffolds with poor mechanical strength and endocytosis-risking broken fibers. Additionally, the mechanisms driving the self-assembly are more intricate, necessitating a complex and meticulous experimental design. In addition to these restrictions, the high cost of synthesis prevents them from being used in regenerative medicine and tissue engineering [97].
Thermally induced phase separation: A homogeneous polymer solution experiences a thermal energy differential during quenching, which starts the void formation. High temperatures solubilize the polymer, and then the temperature is rapidly lowered to trigger phase separation. The solution then separates into a solvent-free phase and a phase free of polymers through vigorous fluid de-blending. This method can adjust the polymer characteristics, solvents, and working temperature to alter the scaffold’s microstructure [98]. This method gives better mechanical qualities with a controlled porous structure and can be compatible with many other manufacturing procedures. Chitosan-magnesium-based composite scaffolds were successfully synthesized by Adhikari et al. through thermally induced phase separation [99]. The platforms had consistent porosity with 50–250 m–sized pores that were tightly linked. Elastic moduli of up to 5 MPa and compressive strengths of up to 400 kPa were found. The in vitro testing revealed that the scaffolds held on to their original three-dimensional frameworks and were unharmed. When exposed to these scaffolds, the 3T3 fibroblast and osteoblast cells showed no cytotoxicity.
Melt molding: Melt-based fabrication techniques have their roots in traditional polymer manufacturing methods. To create porous material, pore-generating techniques can be used [100]. It is common practice to combine water-soluble salts with the polymer during molding and dissolve them in water afterward to produce porous structures. The benefits of this approach include the avoidance of hazardous solvents and the ability to regulate pore size by utilizing porogens of the appropriate size [100]. This method exhibits significant advantages for creating ECM-mimetic tissue regeneration studies by integrating techniques including particle leaching, gas foaming, and the usage of porogens. Dutta et al. created porosity in magnesium scaffolds by using spherical naphthalene particles as the porogen in a powder metallurgy process [101]. Porogen was eliminated by heating at 120 °C for 24 h; then the material was sintered in an argon environment for 2 h at 550 °C. The scanning data show that scaffolds have connected porous structures with a pore size of around 60 µm. The scaffolds’ compressive strength was determined to be between 24 and 184 MPa, and it dropped as the porogen concentration rose. A study of in vitro degradation in phosphate-buffered saline (PBS) revealed that the porosity content of the scaffold controlled how quickly it degraded.
Solvent casting: One of two methods for creating scaffolds via solvent casting is based on the evaporation feature of particular solvents. One method involves dipping a mold into the polymer solution and giving it enough time to draw off the solvent so that the polymeric membrane layer can form. The alternative method involves pouring the polymer solution into a mold and giving it enough time to evaporate so that a layer of the membrane adheres to the mold. This method requires no specialist equipment, is relatively straightforward to use, is affordable, and has minimal effects on degrading behavior. However, it employs highly hazardous solvents that can denature proteins and other integrated compounds, but this can be avoided by letting the scaffold completely dry out utilizing a vacuum procedure. Some researchers have coupled solvent casting with other approaches, such as salt leaching, to increase the scaffold’s features while avoiding the solvent casting’s drawback approaches, such as salt leaching. This method, meanwhile, is only effective when building very thin scaffolds. Solvent casting and salt leaching procedures were used to create porous metallic magnesium/PLGA scaffolds [102]. It was observed that adding various amounts of magnesium to PLGA scaffolds improved their compressive strength and modulus while also creating a porous structure ideal for cell infiltration. Additionally, a pH buffering effect and long-term magnesium relation of a 10-week degradation trial were obtained by reacting basic-degrading magnesium with acidic-degrading PLGA. Micro-CT and histological examination of the magnesium/PLGA scaffolds revealed that they were safer and more efficient in preserving bone height than empty controls. This study showed that 3D magnesium/PLGA composite scaffolds show potentially promising applications for orthopedic bone regeneration.
This entry is adapted from the peer-reviewed paper 10.3390/polym14245460
This entry is offline, you can click here to edit this entry!
