Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Langerhans cells (LCs) constitute a cellular immune network across the epidermis. Because they are located at the skin barrier, they are considered immune sentinels of the skin. These antigen-presenting cells are capable of migrating to skin draining lymph nodes to prime adaptive immune cells, namely T- and B-lymphocytes, which will ultimately lead to a broad range of immune responses. LCs have been shown to possess important roles in the anti-cancer immune responses.
- Langerhans cell
- inflammation
- skin pathology
1. Introduction
Langerhans cells (LCs) are ontogenetically tissue resident macrophages that are functionally more similar to dendritic cells (DCs). In their steady state, they are found mainly in the epidermis, and after activation, they can migrate to skin draining lymph nodes where they play the role of antigen presenting cells (APCs). LCs display important roles in both the stimulation and suppression of adaptive immune responses. After uptake, LCs process antigens into peptides that they then present via MHC molecules to either naïve T cells in the skin draining lymph nodes or to memory T cells in the skin. LCs can activate T cells to produce various adaptive immune responses, including Th1, Th2, Th17 immune responses, as well as immunosuppression via the expansion of regulatory T cells (Tregs). Figure 1 depicts the functions of LCs in the skin.
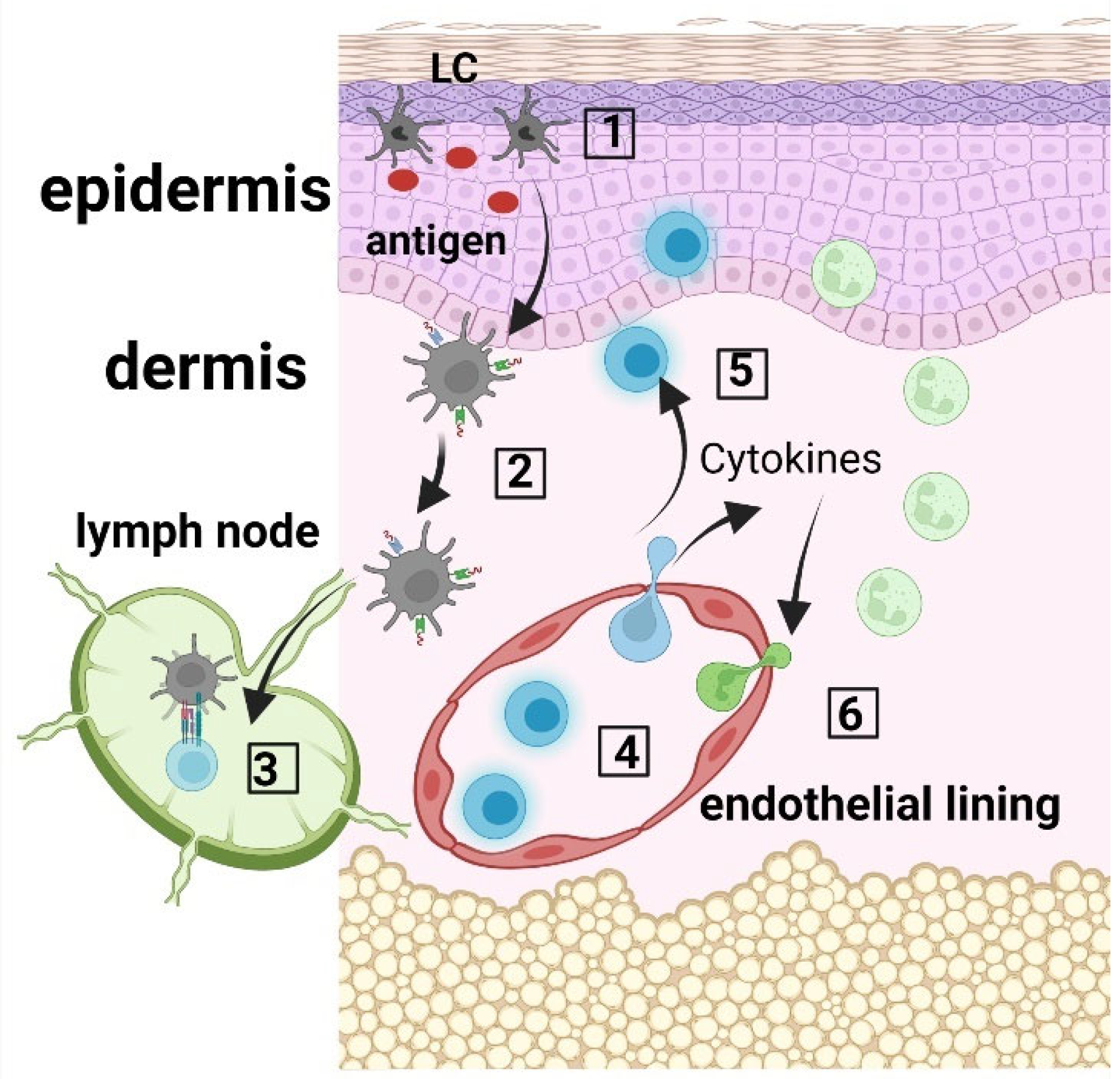
Figure 1. LC immune network. (1) Langerhans cells (LCs) within the epidermis take up antigen; (2) LCs internalize and process the antigen, and present the produced peptides on their surface via MHC molecules; (3) LCs migrate to the skin draining lymph nodes where they prime CD45RA+ naïve T cells; (4) Activated T cells migrate to the skin, where they produce cytokines and chemokines (5), which recruit innate immune cells, including neutrophils from the blood circulation (6).
In the steady state, the renewal of LCs in the epidermis is mediated via the local proliferation of skin resident LCs. Indeed, mature LCs residing in the skin possess an intrinsic ability to enter a cell division cycle, when required, hence allowing the replenishment of the LC population in the epidermis [1]. LCs constitute an immune network across the epidermis and, in turn, serve as skin immune sentinels [2]. Thus, in inflammatory conditions, LCs continuously migrate to the skin draining lymph nodes [3]. When a mass exodus of LCs leads to their numbers being depleted, LC replenishment is ensured by two successive waves of blood-derived cells, i.e., monocytes [4], followed by a myeloid precursor [5][6].
LCs are characterized by the expression of langerin and the presence of Birbeck granules in both mice and humans. However, in vivo mouse experiments have shown that langerin is not a specific marker of LCs because other subsets of DCs, which are located in extra-cutaneous and non-lymphoid tissues, also express langerin [7]. Furthermore, subsets of DCs found in most connective tissues, including the dermis, express langerin. Langerin+ DCs are also positive for CD103 and are CD11blow, in contrast to LCs [8]. LCs express Langerin/CD207, MHC class II, E-Cadherin, EpCAM (Epithelial Cell Adhesion Molecule)/TROP1, Integrin alpha X/CD11c, and human LCs additionally express CD1a, whereas mouse LCs express CD11b+, CD205+ and F4/80+ [6].
Single-cell RNASeq data and mass spectrometry flow cytometry have recently shown that LCs are not a homologous population in the skin [9][10][11]. These results show that LCs can be subcategorized into two major classes (LC1 and LC2); these cell types are either localized to the skin, or are activated and migratory cell types in transit from the epidermis. In general, LC1 are specialized toward antigen uptake and cytokine secretion related to innate immunity, while adaptive and tolerogenic responses are better initiated by LC2 cells. These subsets have only recently been defined; therefore, their exact role in the development of skin pathologies has not been investigated in detail, but it might account for some of the discrepancies encountered when investigating the role of LCs in skin diseases.
2. Role of Langerhans Cells in Wound Healing
Many skin injuries, such as wounds, provoke the migration of LCs out of the epidermis. In this context, the repopulation of the tissue is first ensured by circulating monocytes [6], recruited to the epidermis via the expression of chemokine receptors (CCR)-2 and -6. Then, the full replenishment of LCs will progressively be ensured by resident LCs expressing Langerin, CD24, and EpCAM [6]. The full acquisition of proper functionality of these immature LCs requires the keratinocyte-derived interleukin (IL)-34 [12]. In a wound environment, keratinocytes up-regulate MICA B (Major histocompatibility complex class I-related chain B), a NKG2D ligand (lymphocyte activation receptor natural killer group 2D), which results in LC migration, and the recruitment of αβ T cells to the epidermis [13][14]. The continuous cross-talk between keratinocytes and LCs is critically involved in the LC migratory process after skin wounding [15]. This inter-cellular communication is guaranteed by pro-inflammatory cytokines, [e.g., IL-1, granulocyte macrophage colony stimulating factor (GM-CSF), tumor necrosis factor (TNF-α)], which are involved in LC migration [16]. In the inflammatory phase of tissue remodeling, LCs repopulate the epidermis [17]. This is likely facilitated by TNF-α, which up-regulates the expression of surface adhesion molecules [18]. In patients diagnosed with diabetes, cutaneous repair is delayed. When glycaemia is normalized in such patients, the number of LCs increases in the epidermis. In diabetic foot ulcers, therapy can enhance the number of LCs in the epidermis, which is associated with better healing, emphasizing the importance of LCs in the regenerative capacity of the skin after wounding [19].
However, the detailed role of LCs in wound healing is not yet fully deciphered and a better understanding of the importance of LCs in such processes will open new therapeutic avenues in wound-induced acute or chronic inflammation [20].
3. Role of Langerhans Cells in Psoriasis
There are abundant results reporting on the role of LCs in psoriasis [2][21]. In experimental mouse models [22], it was demonstrated that the IL-23/Th17 axis is involved in the psoriasis-like inflammation [23]. LCs [24] or cDCs [25] have been shown to be, alternatively, the major source of IL-23 in this animal model (i.e., topical application of Imiquimod). The animal model of induced psoriasis using the topical application of Imiquimod is a commonly used psoriasis-mouse model, where, after several applications of the compound on the shaved skin psoriatic, lesions appear. These skin inflammation lesions are severe and animals can be scored as psoriasis patients using individual PASI scores (e.g., erythema, thickening and skin scaling) [22]. The fact that published reports show discrepancies regarding the IL-23 cellular source, is explained by experimental details regarding the model, with psoriasis-like inflammation induced on the ear versus on the back of animals. CD1a is a surface molecule expressed at the cell surface of LCs, which presents lipid antigens. When CD1a is over-expressed, symptoms of psoriasis are aggravated in the skin. In line with this, in psoriatic patients, inflammation and amounts of cytokines in the skin, especially TNF-α, IL-1β [26][27] and IL-17α [28], are lowered by treatment with anti-CD1a antibodies [29].
In a recent work, it was shown that diminished secretion of IL-23 by LCs reduced the interaction between LCs and γδ T cells, thereby inhibiting γδ T cells and, in turn, IL-17 secretion, ultimately leading to improvements in psoriasis-like symptoms in a murine model [30]. Interestingly, in cancer patients with immune-related adverse events (irAE), the development of psoriasis is associated with the loss of epidermal CD1a+ LCs after treatment with immune checkpoint inhibitors (ICI). It is likely that the ICI-therapy aggravates a pre-existing psoriasis phenotype in some cancer patients with low numbers of LCs in the epidermis [31]. In another recent study, it was shown that TGFβ is important in maintaining functional LCs and memory T cells in the skin. Moreover, authors show that integrins avβ6 and avβ8, which are able to activate latent TGFβ, were increased in human keratinocytes from psoriatic skin, which might contribute to maintaining cutaneous inflammation [32]. We have shown increased numbers of epidermal LCs with predominant basal/suprabasal, as well as dermal location, in psoriatic skin lesions (Figure 2).
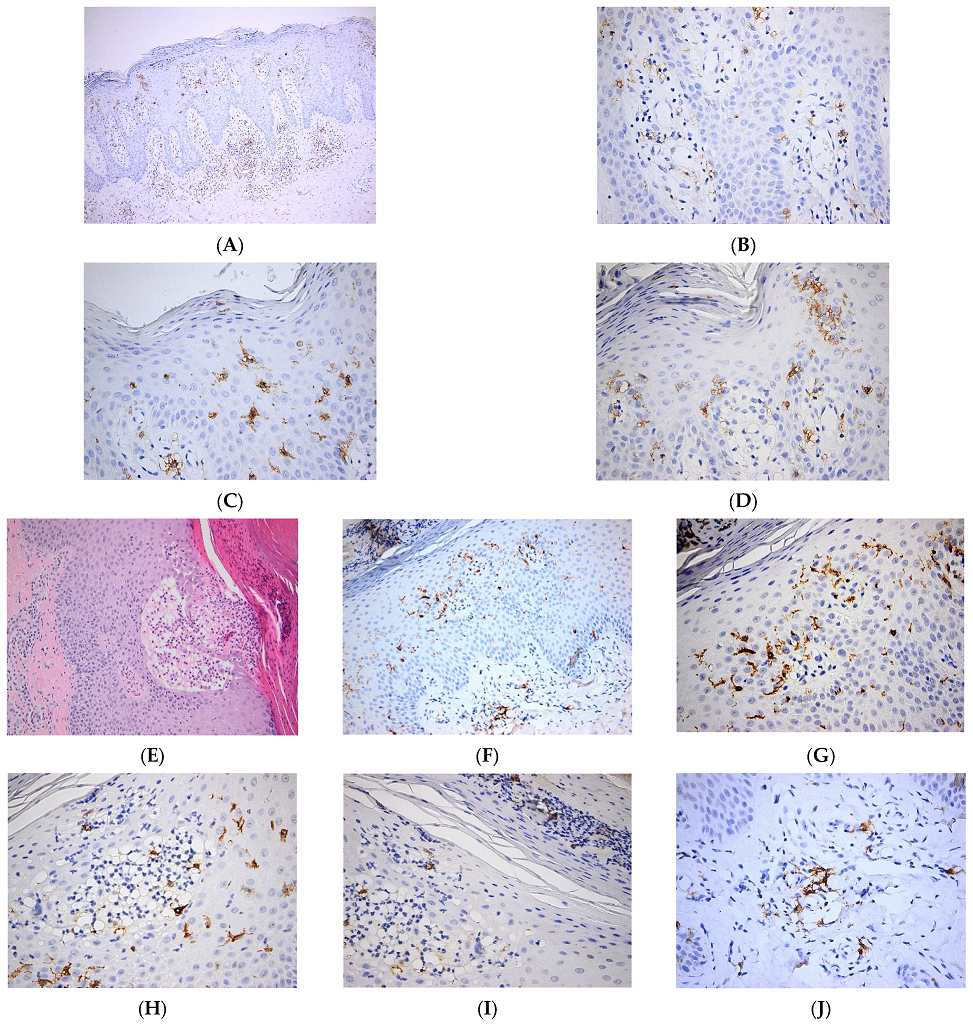
Figure 2. Langerhans cell distribution in psoriatic skin lesions. (A). Numerous Langerhans cells are present both within the epidermis and dermis. CD1a × 100. (B). Numerous Langerhans cells with perivascular distribution in papillary dermis. CD1a × 400. (C). Frequent intraepidermal Langerhans cells with diffuse distribution in the whole epidermal layer. Occasional Langerhans cells within the parakeratotic layer. CD1a × 400. (D). Frequent intraepidermal Langerhans cells with a predominantly basal/suprabasal location. Few Langerhans cells present in the corneous layer. CD1a × 400; (E–J). Pustulous psoriasis. (E). Thick epidermis with subcorneous vesicle filled by serum containing neutrophils; suprajacent agranulosis; marked parakeratosis with neutrophilic abscess; keratinocytic proliferation HE × 100. (F). Numerous Langerhans cells within the epidermis and papillary dermis. CD1a × 100. (G). Numerous Langerhans cells with predominantly basal/suprabasal location. CD1a × 400. (H). Numerous intraepidermal Langerhans cells are present both within the subcorneous vesicle and in the adjacent epidermis. CD1a × 400. (I). Frequent intraepidermal Langerhans cells present in the parakeratotic corneous layer. CD1a × 400. (J). Perivascular Langerhans cells in papillary dermis. CD1a × 400.
4. The Role of Langerhans Cells in Other Inflammatory Skin Pathologies
A pathology with clear LC involvement is dermatopathic lymphadenopathy. This disease starts in the skin lymph nodes, which drains the sites of chronic cutaneous diseases, ranging from infections to neoplasms. A recent study has shown in several dermatological disorders (i.e., mycosis fungoides, chronic inflammatory dermatoses, melanoma, squamous cell carcinoma and Kaposi sarcoma upon human immunodeficiency virus infection), the presence of three types of DC subsets in the paracortical regions of lymph nodes. These APCs were immunophenotypically identified as LCs (S100+, CD1ahigh, langerin+), interdigitating DCs (S100+, CD1alow, langerin−) and a minor DC subpopulation (S100+, CD1a−, langerin−). Moreover, the localization of langerin+ cells via IHC in the trabecular and medullary regions can contribute to ameliorating the differential diagnosis in LCH [33].
The increasing rate of infection with genus beta human papillomaviruses (HPV) [34], which has been shown to be involved in several cancers, including non-melanoma skin cancer (NMSC), raises concerns in the whole population. Ten years ago, the first case of HPV infection in patients diagnosed with epidermodysplasia verruciformis (EV), was reported. Interestingly, LCs are consistently lacking in HPV8+ skin samples from EV patients. It was shown that the differentiation-associated transcription factor CCAAT/enhancer binding protein β (C/EBPβ), which is a target of HPV8 E7 oncoprotein, critically controls the expression of the CCL20 gene in keratinocytes. Thus, HPV8 E7 suppresses the C/EBPβ-inducible and constitutive expression of CCL20, and hinders LC migration [35].
LCs express a receptor that recognizes the presence of double-stranded DNA of microbial or host cellular origin, called absent in melanoma 2 (AIM2). AIM2 induces IL-1β via the activation of the inflammasome. In inflammatory skin disorders, such as psoriasis and atopic dermatitis, and in experimental wounds or chronic venous leg ulcers, the upregulation of AIM2 in LCs might significantly contribute to pathomechanisms by enhancing the production of inflammatory cytokines, such as IL-1β, able to activate surrounding keratinocytes [36].
The role of LCs in atopic dermatitis has been extensively studied and reviewed elsewhere [37][38][39][40].
This entry is adapted from the peer-reviewed paper 10.3390/jpm12122072
References
- Collin, M.; Milne, P. Langerhans cell origin and regulation. Curr. Opin. Hematol. 2016, 23, 28–35.
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells as Immune Regulators within the Skin. Front Immunol. 2018, 8, 1941.
- Geissmann, F.; Dieu-Nosjean, M.C.; Dezutter, C.; Valladeau, J.; Kayal, S.; Leborgne, M.; Brousse, N.; Saeland, S.; Davoust, J. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 2002, 196, 417–430.
- Ginhoux, F.; Tacke, F.; Angeli, V.; Bogunovic, M.; Loubeau, M.; Dai, X.M.; Stanley, E.R.; Randolph, G.J.; Merad, M. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006, 7, 265–273.
- Nagao, K.; Kobayashi, T.; Moro, K.; Ohyama, M.; Adachi, T.; Kitashima, D.Y.; Ueha, S.; Horiuchi, K.; Tanizaki, H.; Kabashima, K.; et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012, 13, 744–752.
- Sere, K.; Baek, J.H.; Ober-Blobaum, J.; Muller-Newen, G.; Tacke, F.; Yokota, Y.; Zenke, M.; Hieronymus, T. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 2012, 37, 905–916.
- Merad, M.; Ginhoux, F.; Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008, 8, 935–947.
- Raaby, L.; Rosada, C.; Langkilde, A.; Lauridsen, K.L.; Vinter, H.; Ommen, P.; Kjellerup, R.B.; Johansen, C.; Iversen, L. Langerhans cell markers CD1a and CD207 are the most rapidly responding genes in lesional psoriatic skin following adalimumab treatment. Exp. Derm. 2017, 26, 804–810.
- Liu, X.; Zhu, R.; Luo, Y.; Wang, S.; Zhao, Y.; Qiu, Z.; Zhang, Y.; Liu, X.; Yao, X.; Li, X.; et al. Distinct human Langerhans cell subsets orchestrate reciprocal functions and require different developmental regulation. Immunity 2021, 54, 2305–2320.e11.
- Reynolds, G.; Vegh, P.; Fletcher, J.; Poyner, E.F.M.; Stephenson, E.; Goh, I.; Botting, R.A.; Huang, N.; Olabi, B.; Dubois, A.; et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 2021, 371, eaba6500.
- Cheng, J.B.; Sedgewick, A.J.; Finnegan, A.I.; Harirchian, P.; Lee, J.; Kwon, S.; Fassett, M.S.; Golovato, J.; Gray, M.; Ghadially, R.; et al. Transcriptional Programming of Normal and Inflamed Human Epidermis at Single-Cell Resolution. Cell Rep. 2018, 25, 871–883.
- Wang, Y.; Szretter, K.J.; Vermi, W.; Gilfillan, S.; Rossini, C.; Cella, M.; Barrow, A.D.; Diamond, M.S.; Colonna, M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012, 13, 753–760.
- Strid, J.; Roberts, S.J.; Filler, R.B.; Lewis, J.M.; Kwong, B.Y.; Schpero, W.; Kaplan, D.H.; Hayday, A.C.; Girardi, M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 2008, 9, 146–154.
- Jung, H.; Hsiung, B.; Pestal, K.; Procyk, E.; Raulet, D.H. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J. Exp. Med. 2012, 209, 2409–2422.
- Strid, J.; Sobolev, O.; Zafirova, B.; Polic, B.; Hayday, A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 2011, 334, 1293–1297.
- Kimber, I.; Cumberbatch, M.; Dearman, R.J.; Bhushan, M.; Griffiths, C.E. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br. J. Derm. 2000, 142, 401–412.
- Juhász, I.; Simon, M., Jr.; Herlyn, M.; Hunyadi, J. Repo.opulation of Langerhans cells during wound healing in an experimental human skin/SCID mouse model. Immunol. Lett. 1996, 52, 125–128.
- Johnson, L.A.; Clasper, S.; Holt, A.P.; Lalor, P.F.; Baban, D.; Jackson, D.G. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006, 203, 2763–2777.
- Stojadinovic, O.; Yin, N.; Lehmann, J.; Pastar, I.; Kirsner, R.S.; Tomic-Canic, M. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol. Res. 2013, 57, 222–228.
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93.
- Antal, D.; Alimohammadi, S.; Bai, P.; Szöllősi, A.G.; Szántó, M. Antigen-Presenting Cells in Psoriasis. Life 2022, 3, 234.
- Surcel, M.; Huică, R.I.; Munteanu, A.N.; Isvoranu, G.; Pîrvu, I.R.; Ciotaru, D.; Constantin, C.; Bratu, O.; Căruntu, C.; Neagu, M.; et al. Phenotypic changes of lymphocyte populations in psoriasiform dermatitis animal model. Exp. Med. 2019, 17, 1030–1038.
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845.
- Yoshiki, R.; Kabashima, K.; Honda, T.; Nakamizo, S.; Sawada, Y.; Sugita, K.; Yoshioka, H.; Ohmori, S.; Malissen, B.; Tokura, Y.; et al. IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing gammadelta T cells. J. Investig. Derm. 2014, 134, 1912–1921.
- Wohn, C.; Ober-Blöbaum, J.L.; Haak, S.; Pantelyushin, S.; Cheong, C.; Zahner, S.P.; Onderwater, S.; Kant, M.; Weighardt, H.; Holzmann, B.; et al. Langerin(neg) conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 10723–10728.
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509.
- Singh, T.; Zhang, H.; Borek, I.; Wolf, P.; Hedrick, M.N.; Singh, S.P.; Kelsall, B.L.; Clausen, B.E.; Farber, J.M. Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nat. Commun. 2016, 7, 13581.
- Gordon, K.B.; Colombel, J.F.; Hardin, D.S. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2016, 375, 2102.
- Kim, J.H.; Hu, Y.; Yongqing, T.; Kim, J.; Hughes, V.A.; Le Nours, J.; Marquez, E.A.; Purcell, A.W.; Wan, Q.; Sugita, M.; et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 2016, 17, 1159–1166.
- Liu, L.; Chen, X.; Lu, Y.; Sun, X.Y.; Ze, K.; Zhou, Y.Q.; Li, W.; Li, X.; Li, H.J.; Li, B. Celastrol gel ameliorates imiquimod-induced psoriasis-like dermatitis in mice by targeting Langerhans cells. Biomed. Pharmacother. 2022, 147, 112644.
- Kase, M.; Fujita, Y.; Ota, A.; Shimizu, S.; Itoi-Ochi, S.; Sano, S. Loss of epidermal Langerhans cells in psoriasiform lesions of de novo induced or worsened pre-existing psoriasis following uses of immune checkpoint inhibitors. J. Dermatol. 2022, 49, 916–920.
- Nguyen, B.A.; Ho, J.; De La Cruz Diaz, J.S.; Nishimura, S.; Kaplan, D.H. TGFβ activating integrins β6 and β8 are dysregulated in inflammatory skin disease and cutaneous melanoma. J. Derm. Sci. 2022, 106, 2–11.
- Garces, S.; Yin, C.C.; Miranda, R.N.; Patel, K.P.; Li, S.; Xu, J.; Thakral, B.; Poppiti, R.J.; Medina, A.M.; Sriganeshan, V.; et al. Clinical, histopathologic, and immunoarchitectural features of dermatopathic lymphadenopathy: An update. Mod. Pathol. 2020, 33, 1104–1121.
- Boda, D.; Neagu, M.; Constantin, C.; Voinescu, R.N.; Caruntu, C.; Zurac, S.; Spandidos, D.A.; Drakoulis, N.; Tsoukalas, D.; Tsatsakis, A.M. HPV strain distribution in patients with genital warts in a female population sample. Oncol. Lett. 2016, 12, 1779–1782.
- Sperling, T.; Ołdak, M.; Walch-Rückheim, B.; Wickenhauser, C.; Doorbar, J.; Pfister, H.; Malejczyk, M.; Majewski, S.; Keates, A.C.; Smola, S. Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012, 8, e1002833.
- de Koning, H.D.; Bergboer, J.G.; van den Bogaard, E.H.; van Vlijmen-Willems, I.M.; Rodijk-Olthuis, D.; Simon, A.; Zeeuwen, P.L.; Schalkwijk, J. Strong induction of AIM2 expression in human epidermis in acute and chronic inflammatory skin conditions. Exp. Dermatol. 2012, 21, 961–964.
- Marschall, P.; Wei, R.; Segaud, J.; Yao, W.; Hener, P.; German, B.F.; Meyer, P.; Hugel, C.; Ada Da Silva, G.; Braun, R.; et al. Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1778–1794.
- Dubrac, S.; Schmuth, M.; Ebner, S. Atopic dermatitis: The role of Langerhans cells in disease pathogenesis. Immunol. Cell Biol. 2010, 88, 400–409.
- Iwamoto, K.; Nümm, T.J.; Koch, S.; Herrmann, N.; Leib, N.; Bieber, T. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR2 activation. Allergy 2018, 73, 2205–2213.
- Kabashima, K. New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. J. Derm. Sci. 2013, 70, 3–11.
This entry is offline, you can click here to edit this entry!
