Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has raised great concerns about human health globally. Prevention and vaccination are still the most efficient ways to slow down the pandemic and to treat SARS-CoV-2 in various aspects.
- SARS-CoV-2
- COVID-19 pandemic
- smart nanostructures
- biosensing
- detection
- vaccination
1. Introduction
The spread of SARS-CoV-2 has caused the coronavirus 2019 (COVID-19) disease and the pandemic worldwide [1][2][3]. According to the weekly report of the World Health Organization (WHO) as of 25 September 2022, 612 million confirmed cases have been reported, leading to 6.5 million deaths by COVID-19 globally. Although the number of newly reported cases decreases by 11% and new weekly reported deaths decrease by 18% compared with the previous week, there is no convincing evidence and global confidence indicating the end of the COVID-19 pandemic so far [4][5][6]. Additionally, the up-to-date death rate caused by the SARS-CoV-2 is 1.06%, which is higher than the 0.6% in the 1957 influenza pandemic, although the latest rates decreased from case fatality rates of 3.3% about two years ago (as of 9 September 2020). In addition to the coronavirus SARS-CoV-2 itself, there has been a trend of outbreaks of various variants globally, making the current situation unpredictable regarding the spreading of the pandemic [7][8][9][10][11]. It is therefore becoming increasingly important to develop biosensing strategies as well as reliable coronaviral vaccines to prevent and treat the SARS-CoV-2 and its variants of concerns [12].
In many established strategies for coronavirus regulation, nanostructured materials that can actively respond to external stimuli are playing increasingly important roles [13][14][15]. Their unique capabilities to sense and specifically respond to external physical and chemical stimuli represent widely accessible platforms to develop active smart coatings for virus prevention, to be incorporated to advanced sensing devices for coronavirus detection, and to be used for responsive delivery systems for SARS-CoV-2 vaccination [16][17][18]. They have demonstrated their great global success in many aspects in fighting against the COVID-19 pandemic. For example, some emerging techniques have been proposed to functionalize conventional masks with active nanostructures [19]. These innovations are expected to improve prevention efficacy of masks in social activities. Another promising example is the use of photonic crystals and plasmonic nanostructures in developing high-performance virus biosensors [20][21][22]. These responsive nanostructured materials can provide both colorimetric changes for fast, point-of-care detection and spectroscopic readouts for precise quantitative evaluation. In developing coronavirus vaccinations, nanostructured materials have been used to deliver biological species for triggering in vivo immune responses. The introduction of lipid nanostructures as a biocompatible carrier to deliver RNA has been used in commercial vaccines, which have demonstrated the highest efficacy based on clinical data [23][24][25]. Considering these exciting developments, it is critical to summarize the design principles and working mechanisms of this unique set of nanostructured materials in coronavirus regulation. Although there are some reviews providing an overview of materials science in fighting against COVID-19 or summarizing research activities in specific aspects (sensing or vaccination) [26][27][28][29], a focused review on recent advances in creating smart nanostructured materials for the SARS-CoV-2 treatment is necessary to understand the general concepts underlying these remarkable materials and to overcomes existing challenges in tackling COVID-19.
2. SARS-CoV-2 and VOCs
The structure of the RNA virus SARS-CoV-2 is depicted in Figure 1a, with its viral RNA encapsulated in the membrane protein [30][31][32]. It comprises five basic functional structures: a spike protein, envelop protein, membrane protein, nucleocapsid protein, and the viral RNA ranking from the exterior to the interior. More specifically, the SARS-CoV-2 is a positive-strand RNA virus (+ssRNA virus), which contains ~29-kilobase single-stranded, positive-sense genomes made of ribonucleic acid. The spike protein known as S protein on the surface regulates the receptor recognition and cell membrane fusion and therefore is one of the most important functional proteins of the virus [7][33][34]. It has two subunits, S1 and S2 on the virus membrane, with a total number of amino acids larger than 1200. The S1 subunit contains a domain that can recognize and bind to the receptor angiotensin-converting enzyme 2. The S2 subunit is responsible for cell membrane fusion through the formation of a six-helical bundle based on a two-heptad repeat domain [35]. Therefore, the S protein has been extensively studied so far for developing vaccines for tackling the coronavirus pandemic, for investigating immune responses, and for tracking genetic mutations among various variants [36][37][38]. Among these complex structures and diverse amino acid constituents, only a small amino acid stretch is directly related to the interactions between the receptor-binding domain and the enzyme 2 receptor of the host cells. Figure 1b shows the key mutations on the S protein that are noted in all VOC so far, indicating the important role of the S protein in virus mutation and vaccination.
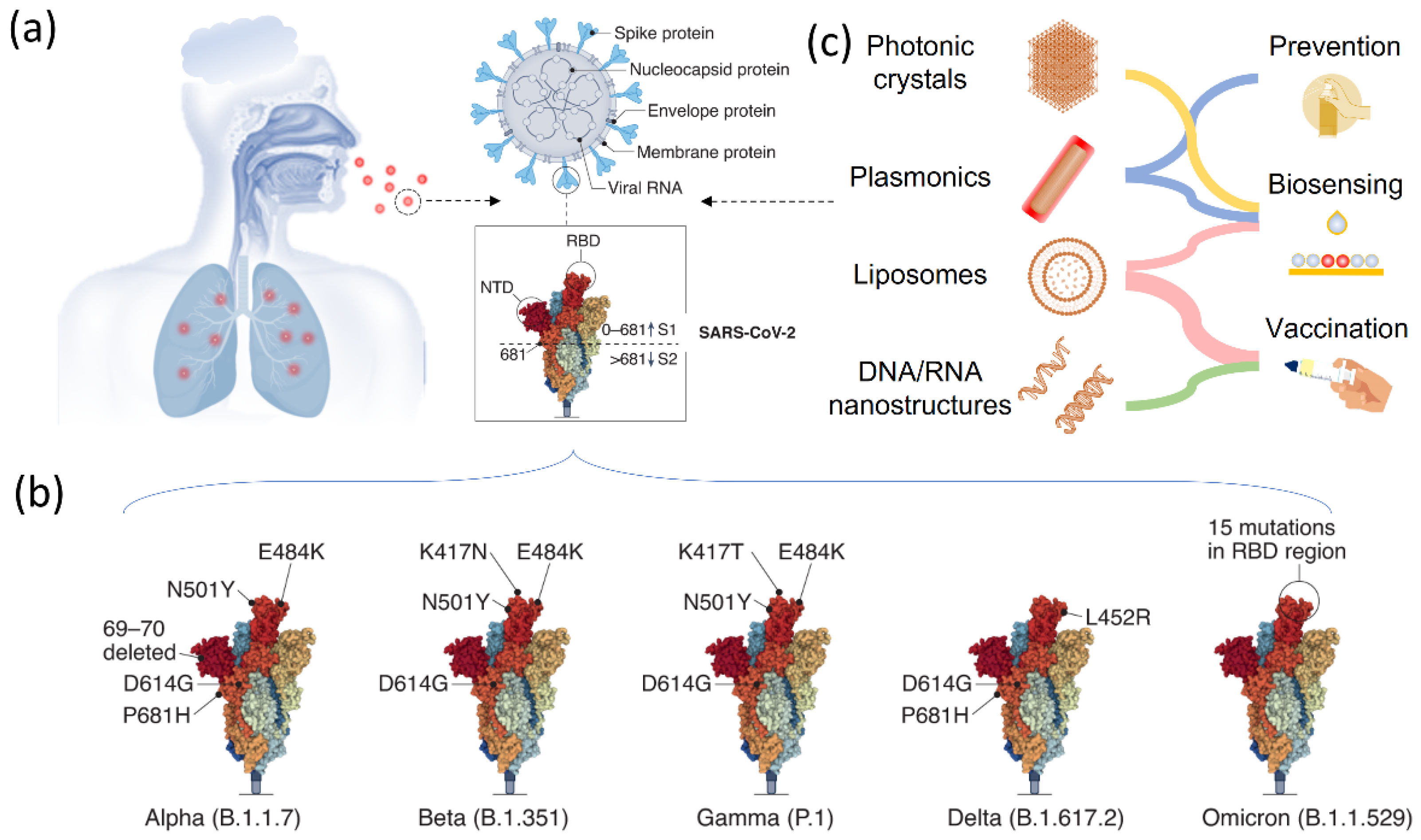
Figure 1. Smart nanostructured materials for SARS-CoV-2 and variants biosensing, treatment, and vaccination. (a) Scheme of smart nanostructured materials for COVID-19 treatment. (b) The structure of SARS-CoV-2 and the major components, including the S protein, nucleocapsid protein, envelope protein, membrane protein and viral RNA. (c) SARS-CoV-2 variants of concerns. Reprinted from [30], with permission from Springer Nature (Berlin/Heidelberg, Germany).
Compared with other RNA virus, SARS-CoV-2 has a slower mutation rate, with two single-letter mutations per month [39][40]. However, due to the rapid spread and large number of infected patents, a variety of variants have been observed globally as SARS-CoV-2 continues to change the vital genetic codes through genetic mutations and viral combinations when replicating their genomes [41][42]. One remarkable feature of these existing variants is that they have one or more genetic mutations being different from the SARS-CoV-2 and the other variants. If a variant evolves through the combination of genetic codes from two different variants, it can be categorized as a recombinant. Although there are many variants, a lineage can sometimes be recognized, which contains virus variants derived from the same ancestor. According to WHO, more than 4000 variants of SARS-CoV-2 have been reported [43]. The major concern regarding these variants is the escape or hamper of these virus variants from the established immune responses by previous infection or SARS-CoV-2 vaccination [44][45][46]. Based on the genetic modification of the various SARS-CoV-2 variants, they can be divided into three categories to facilitate the necessary attention for the policy determination and efficient treatment, including variants under monitoring, variants of interest (VOI), and variants of concerns (VOC). More specifically, these three types of virus variants are ranked based on the virus genetic changes that are predicted or known to alter a few important properties of the coronavirus, including transmissibility, disease severity, immune responses, and the therapeutic and diagnostic outcomes. Depending on their genetic mutations and the virus spread, it may vary significantly over time.
Understanding the genetic information and biological properties is a prerequisite for developing effective administration strategies and vaccination for SARS-CoV-2 and its variants. To this end, responsive or smart nanostructured materials are playing an important role in different aspects. As shown in Figure 1c, various nanostructured materials, including photonic crystals, plasmonic nanostructures, lipid nanoparticles, and DNA/RNA nanostructures have been studied and used in research and in clinics for the prevention, biosensing, and vaccination of SARS-CoV-2 and its variants. In response to external stimuli or surrounding environmental changes, these materials can show perceivable or detectable signals, which represents an open platform for developing high-performance sensors [47][48][49]. Plasmonic nanostructures and photonic crystals are two representative materials in this regard, which offer programmable optical signals for the sensing and detection of the virus and biomolecules [50][51][52][53][54]. Therefore, they have been extensively used for detecting SARS-CoV-2 and its various variants in current research. One remarkable feature of these nanostructured optical sensors is the readable outputs and colorimetric sensing in response to virus exposure, which significantly facilitates point-of-care and fast detection of the SARS-CoV-2 in a flexible time scale. Moreover, they do not require additional energy input to perform the test, which greatly extends the availability of these biosensors in daily use. Performing quantitative analysis is also possible on these optical sensors by taking advantage of various spectroscopies. Another great success in fighting against the SARS-CoV-2 is the use of nanostructures for virus vaccination, which delivers biological molecules to trigger the immune reactions inside the body. To this end, cationic lipids containing nucleic acids and virus-mimicking nanoparticles for accomplishing S protein delivery are two remarkable examples, demonstrating at the level of fundamental research and clinical trials the great success of nanostructured vaccines in bringing the coronavirus under control, in preventing viral infection, and in reducing disease severity [55].
3. Nanostructured Materials for COVID-19 Prevention
One effective way to slow down virus spread is to physically isolate the infectious viruses that are suspended in air. A few common practices nowadays include keeping social distance and wearing personal protective equipment (masks, gloves, face shields, and protective suits). In addition to these common practices, researchers are seeking ways to prevent the spread of the coronavirus using nanostructured filters or coatings, which aim to reduce the number of virus particles suspended in air by capturing them on demand. In the classic design of air filters, filters with regular pores allow selective transport to particulate matters of different sizes. Only particulate matters or nanoscale particles with sizes smaller than the pore diameters can pass through the filters, leaving larger ones blocked and separated. This working mechanism is operational for particulate matters as well as biological species. Functionalizing the filtering materials represents an advanced technique to improve the efficiency [56][57][58]. For example, the top-down fiber manufacturing is a typical method to prepare functional filters, which can be explained by a Brownian diffusion mechanism [59][60][61]. An Al-coated conductive fibrous filter demonstrated an efficiency of >99.99% nanoparticle capture by using electrostatic interactions [62]. However, these strategies require the additional integration of a nanogenerator set and some filters also need ultra-high voltage, which limits their practical use. To overcome these existing challenges, a self-powered filter based on ionic liquid polymer composites was developed with improved hydrophilicity and conductivity, high absolute electrostatic potential, and power generation ability to remove nanoparticles and particulate matters [63]. This self-powered filter was prepared by polymerizing a hydrophilic copolymer on a melamine-formaldehyde (MF) resin sponge. Such highly porous structures allow polluted air to flow through the filter without too much pressure drop while enhancing the particle and virus-removal efficiency, owning to their high surface areas and porosity. This filter demonstrates a high efficiency in removing particulate matters by generating a strong electric field under a low voltage of 3 V. Such a low voltage could be supplied by a silicon solar panel, granting this filter great potential in creating self-powered wearable cleaning devices. Functionalizing nanofibers with active nanostructures will provide additional antibacterial and antiviral properties in addition to passive filtration [64][65][66][67]. To this end, Ag nanoparticles have been long recognized for their excellent antibacterial performances [68][69][70][71]. A typical scheme for classic air filter with antibacterial and antiviral properties is shown in Figure 2a [72]. In this work, a polar polymer, PA6, was made into nanofibers by electrospinning and deposited on a polypropylene substrate. Ag nanoparticles were decorated on the fibrous membranes through a impregnation method (SEM in Figure 2b). Such nanostructured films were used as active filters, which removed suspended bacteria and virus based on the antibacterial and antiviral properties of the guest Ag nanoparticles (Figure 2c).
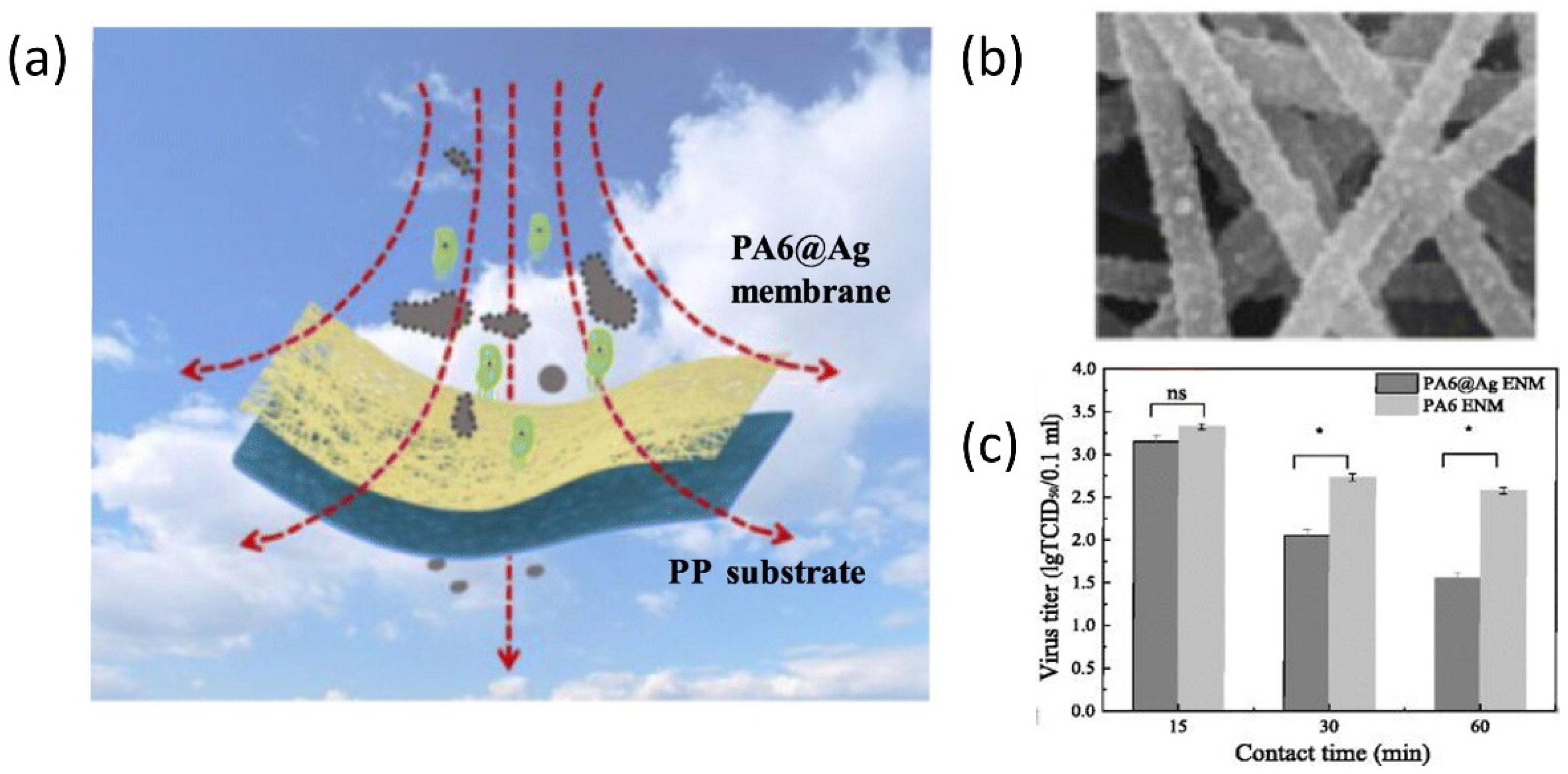
Figure 2. Responsive nanostructured materials for potential COVID-19 treatment and prevention. (a) Schematic illustration of nanostructured filter. (b) The SEM image of the nanostructured fibers decorated with Ag nanoparticles. (c) The viral titer measurement at different contact time. ns: not significant difference; * p < 0.05. Reprinted from [72], with permission from Elsevier Inc (Amsterdam, The Netherlands).
Another technique that has been established to remove virus or particles is to actively capture these pollutants in the air with smart coatings [73][74]. The purpose of this design is to remove pathogen-laden respiratory droplets released from nearby patients, which is expected to slow down the spread of pathogens and reduce the transmission of coronavirus. To this end, a cosmetic ingredient-based formulation has been reported to form conformal coatings on surfaces of different materials, compositions, shapes, roughness, and wettability, which can enhance the aerosol-capturing capability [75]. This work introduced polyelectrolytes as coating materials that can increase the wettability by the droplets, delay the elastic recovery of deformed droplets for enhanced deposition, and absorb water quickly from the captured droplets to avoid releasing. To demonstrate these effects, an analytical model was built, which used air-spray to mimic droplet formation (Figure 3a). This quantitative modal demonstrates an enhanced efficiency for droplet-capturing, since the coated surfaces had a lower count of droplets of different sizes (Figure 3b).
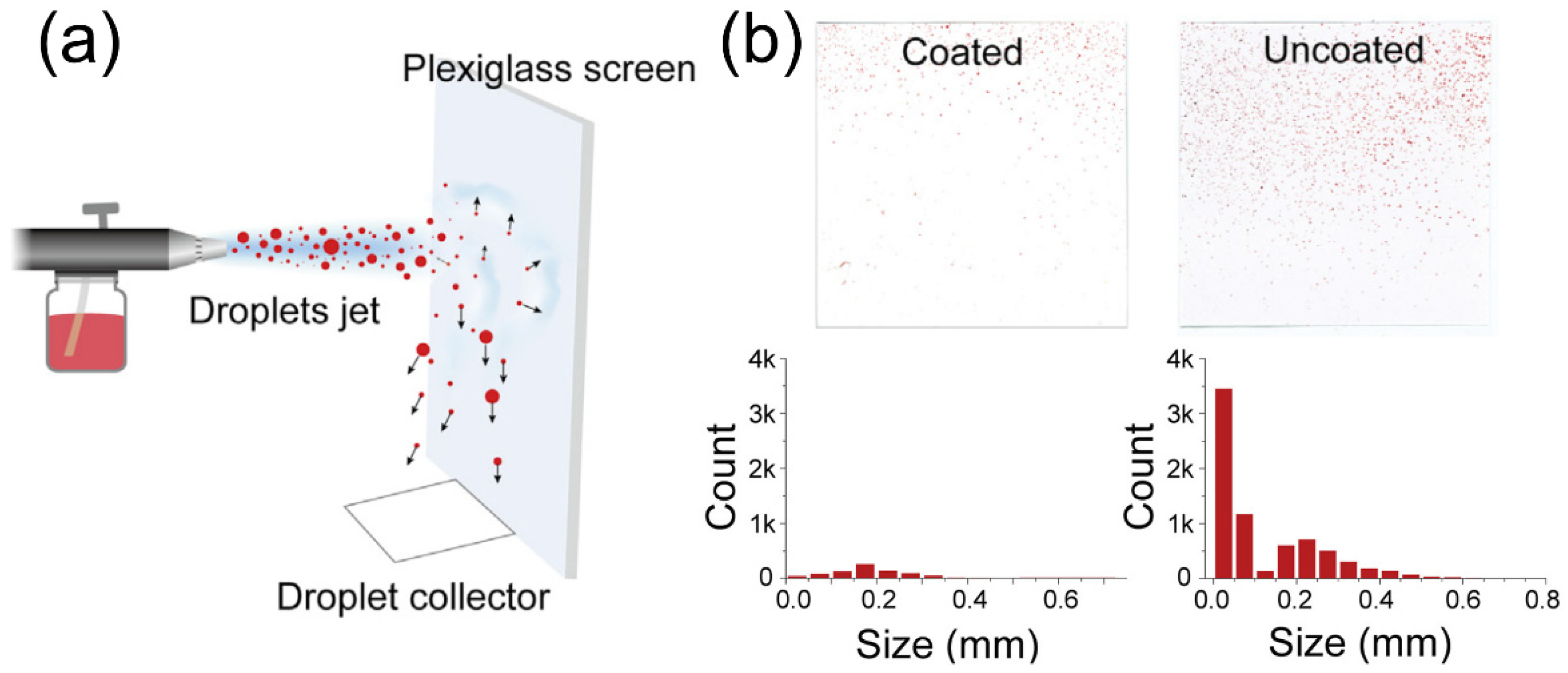
Figure 3. Nanostructured coating for COVID-19 treatment and prevention. (a) Schematic illustration of spray experiment for accessing the treatment performance. (b) Photos and corresponding histograms showing the droplet marks and their size distributions, respectively. Reprinted from [75], with permission from Elsevier Inc.
This entry is adapted from the peer-reviewed paper 10.3390/bios12121129
References
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527.
- Lippi, G.; Mattiuzzi, C.; Henry, B.M. Updated picture of SARS-CoV-2 variants and mutations. Diagnosis 2021, 9, 11–17.
- Drain, P.K. Rapid diagnostic testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272.
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302.
- Ozili, P.K.; Arun, T. Spillover of COVID-19: Impact on the Global Economy. In Managing Inflation and Supply Chain Disruptions in the Global Economy; IGI Global: Hershey, PA, USA, 2022; pp. 41–61.
- Hasan, I.; Dhawan, P.; Rizvi, S.; Dhir, S. Data analytics and knowledge management approach for COVID-19 prediction and control. Int. J. Inf. Technol. 2022, 1–18.
- Hadj Hassine, I. COVID-19 vaccines and variants of concern: A review. Rev. Med. Virol. 2022, 32, e2313.
- Sagulkoo, P.; Plaimas, K.; Suratanee, A.; Vissoci Reiche, E.M.; Maes, M. Immunopathogenesis and immunogenetic variants in COVID-19. Curr. Pharm. Des. 2022, 28, 1780–1797.
- Ciotti, M.; Ciccozzi, M.; Pieri, M.; Bernardini, S. The COVID-19 pandemic: Viral variants and vaccine efficacy. Crit. Rev. Clin. Lab. Sci. 2022, 59, 66–75.
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; Moustafa Abo El-Ella, D.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mohsen, M.O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022, 54, 524–540.
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832.
- Rasmi, Y.; Saloua, K.S.; Nemati, M.; Choi, J.R. Recent progress in nanotechnology for COVID-19 prevention, diagnostics and treatment. Nanomaterials 2021, 11, 1788.
- Li, Z.; Yang, F.; Yin, Y. Smart materials by nanoscale magnetic assembly. Adv. Funct. Mater. 2020, 30, 1903467.
- Pishva, P.; Yüce, M. Nanomaterials to tackle the COVID-19 pandemic. Emergent Mater. 2021, 4, 211–229.
- Srivastava, M.; Srivastava, N.; Mishra, P.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2021, 754, 142363.
- Li, Z.; Fan, Q.; Yin, Y. Colloidal self-assembly approaches to smart nanostructured materials. Chem. Rev. 2021, 122, 4976–5067.
- Ghaemi, F.; Amiri, A.; Bajuri, M.Y.; Yuhana, N.Y.; Ferrara, M. Role of different types of nanomaterials against diagnosis, prevention and therapy of COVID-19. Sustain. Cities Soc. 2021, 72, 103046.
- Li, Z.; Wang, C.; Cheng, L.; Gong, H.; Yin, S.; Gong, Q.; Li, Y.; Liu, Z. PEG-functionalized iron oxide nanoclusters loaded with chlorin e6 for targeted, NIR light induced, photodynamic therapy. Biomaterials 2013, 34, 9160–9170.
- Abbasinia, M.; Karimie, S.; Haghighat, M.; Mohammadfam, I. Application of nanomaterials in personal respiratory protection equipment: A literature review. Safety 2018, 4, 47.
- Li, Z.; Wang, X.; Han, L.; Zhu, C.; Xin, H.; Yin, Y. Multicolor Photonic Pigments for Rotation-Asymmetric Mechanochromic Devices. Adv. Mater. 2022, 34, 2107398.
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic crystals for chemical sensing and biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335.
- Joshi, N.; Shukla, S.; Narayan, R.J. Novel photonic methods for diagnosis of SARS-CoV-2 infection. Transl. Biophotonics 2022, 4, e202200001.
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 2021, 55, 2–12.
- Kim, M.; Jeong, M.; Hur, S.; Cho, Y.; Park, J.; Jung, H.; Seo, Y.; Woo, H.; Nam, K.; Lee, K.; et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021, 7, eabf4398.
- Blakney, A.K.; McKay, P.F.; Hu, K.; Samnuan, K.; Jain, N.; Brown, A.; Thomas, A.; Rogers, P.; Polra, K.; Sallah, H.; et al. Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines. J. Control. Release 2021, 338, 201–210.
- Morales-Narváez, E.; Dincer, C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens. Bioelectron. 2020, 163, 112274.
- Tang, Z.; Kong, N.; Zhang, X.; Liu, Y.; Hu, P.; Mou, S.; Liljeström, P.; Shi, J.; Tan, W.; Kim, J.S.; et al. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020, 5, 847–860.
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 2020, 14, 6383–6406.
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martin-Sampedro, R.; Del Real, G.; Aranda, P. Nanotechnology responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979.
- Huang, X.; Kon, E.; Han, X.; Zhang, X.; Kong, N.; Mitchell, M.J.; Peer, D.; Tao, W. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat. Nanotechnol. 2022, 17, 1027–1037.
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science 2022, 375, 864–868.
- Ye, G.; Liu, B.; Li, F. Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain. Nat. Commun. 2022, 13, 1214.
- Du, L.; Yang, Y.; Zhang, X.; Li, F. Recent advances in nanotechnology-based COVID-19 vaccines and therapeutic antibodies. Nanoscale 2022, 14, 1054–1074.
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20.
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149.
- Langel, S.N.; Johnson, S.; Martinez, C.I.; Tedjakusuma, S.N.; Peinovich, N.; Dora, E.G.; Kuehl, P.J.; Irshad, H.; Barrett, E.G.; Werts, A.D.; et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 2022, 14, eabn6868.
- Ng, K.W.; Faulkner, N.; Finsterbusch, K.; Wu, M.; Harvey, R.; Hussain, S.; Greco, M.; Liu, Y.; Kjaer, S.; Swanton, C.; et al. SARS-CoV-2 S2–targeted vaccination elicits broadly neutralizing antibodies. Sci. Transl. Med. 2022, 14, eabn3715.
- Huang, H.-Y.; Liao, H.-Y.; Chen, X.; Wang, S.-W.; Cheng, C.-W.; Shahed-Al-Mahmud, M.; Liu, Y.-M.; Mohapatra, A.; Chen, T.-H.; Lo, J.M.; et al. Vaccination with SARS-CoV-2 spike protein lacking glycan shields elicits enhanced protective responses in animal models. Sci. Transl. Med. 2022, 14, eabm0899.
- Callaway, E. The coronavirus is mutating—Does it matter? Nature 2020, 585, 174–178.
- Williams, T.C.; Burgers, W.A. SARS-CoV-2 evolution and vaccines: Cause for concern? Lancet Respir. Med. 2021, 9, 333–335.
- Ahmad, S.U.; Kiani, B.H.; Abrar, M.; Jan, Z.; Zafar, I.; Ali, Y.; Alanazi, A.M.; Malik, A.; Rather, M.A.; Ahmad, A. A comprehensive genomic study, mutation screening, phylogenetic and statistical analysis of SARS-CoV-2 and its variant omicron among different countries. J. Infect. Public Health 2022, 15, 878–891.
- Escalera, A.; Gonzalez-Reiche, A.S.; Aslam, S.; Mena, I.; Laporte, M.; Pearl, R.L.; Fossati, A.; Rathnasinghe, R.; Alshammary, H.; van de Guchte, A.; et al. Mutations in SARS-CoV-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe 2022, 30, 373–387.e7.
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773.
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424.
- Abdool Karim, S.S.; de Oliveira, T. New SARS-CoV-2 variants—Clinical, public health, and vaccine implications. N. Engl. J. Med. 2021, 384, 1866–1868.
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.-M.; et al. SARS-CoV-2 variants and vaccines. N. Engl. J. Med. 2021, 385, 179–186.
- Li, Z.; Yin, Y. Stimuli-responsive optical nanomaterials. Adv. Mater. 2019, 31, 1807061.
- Yoshida, M.; Lahann, J. Smart nanomaterials. ACS Nano 2008, 2, 1101–1107.
- Aflori, M. Smart nanomaterials for biomedical applications—A review. Nanomaterials 2021, 11, 396.
- Murray, W.A.; Barnes, W.L. Plasmonic materials. Adv. Mater. 2007, 19, 3771–3782.
- Zeng, J.; Gong, M.; Wang, D.; Li, M.; Xu, W.; Li, Z.; Li, S.; Zhang, D.; Yan, Z.; Yin, Y. Direct synthesis of water-dispersible magnetic/plasmonic heteronanostructures for multimodality biomedical imaging. Nano Lett. 2019, 19, 3011–3018.
- Li, Z.; He, L.; Zeng, J. Recent Advances in Responsive Optical Nanomaterials. Front. Chem. 2021, 9, 760187.
- Li, Z.; Fan, Q.; Wu, C.; Li, Y.; Cheng, C.; Yin, Y. Magnetically tunable plasmon coupling of Au nanoshells enabled by space-free confined growth. Nano Lett. 2020, 20, 8242–8249.
- Li, Z.; Wang, W.; Yin, Y. Colloidal assembly and active tuning of coupled plasmonic nanospheres. Trends Chem. 2020, 2, 593–608.
- Nel, A.E.; Miller, J.F. Nano-enabled COVID-19 vaccines: Meeting the challenges of durable antibody plus cellular immunity and immune escape. ACS Nano 2021, 15, 5793–5818.
- Han, K.S.; Lee, S.; Kim, M.; Park, P.; Lee, M.H.; Nah, J. Electrically activated ultrathin PVDF-TrFE air filter for high-efficiency PM1.0 filtration. Adv. Funct. Mater. 2019, 29, 1903633.
- Chen, Y.; Zhang, S.; Cao, S.; Li, S.; Chen, F.; Yuan, S.; Xu, C.; Zhou, J.; Feng, X.; Ma, X.; et al. Roll-to-roll production of metal-organic framework coatings for particulate matter removal. Adv. Mater. 2017, 29, 1606221.
- Gu, G.Q.; Han, C.B.; Lu, C.X.; He, C.; Jiang, T.; Gao, Z.L.; Li, C.J.; Wang, Z.L. Triboelectric nanogenerator enhanced nanofiber air filters for efficient particulate matter removal. ACS Nano 2017, 11, 6211–6217.
- Han, C.B.; Jiang, T.; Zhang, C.; Li, X.; Zhang, C.; Cao, X.; Wang, Z.L. Removal of particulate matter emissions from a vehicle using a self-powered triboelectric filter. ACS Nano 2015, 9, 12552–12561.
- Wang, N.; Yang, Y.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.; Ding, B. Ultra-light 3D nanofibre-nets binary structured nylon 6–polyacrylonitrile membranes for efficient filtration of fine particulate matter. J. Mater. Chem. A 2015, 3, 23946–23954.
- Zhang, S.; Liu, H.; Yin, X.; Yu, J.; Ding, B. Anti-deformed polyacrylonitrile/polysulfone composite membrane with binary structures for effective air filtration. ACS Appl. Mater. Interfaces 2016, 8, 8086–8095.
- Choi, D.Y.; Jung, S.-H.; Song, D.K.; An, E.J.; Park, D.; Kim, T.-O.; Jung, J.H.; Lee, H.M. Al-coated conductive fibrous filter with low pressure drop for efficient electrostatic capture of ultrafine particulate pollutants. ACS Appl. Mater. Interfaces 2017, 9, 16495–16504.
- Zhang, G.-H.; Zhu, Q.-H.; Zhang, L.; Yong, F.; Zhang, Z.; Wang, S.-L.; Wang, Y.; He, L.; Tao, G.-H. High-performance particulate matter including nanoscale particle removal by a self-powered air filter. Nat. Commun. 2020, 11, 1653.
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077.
- Liu, Y.; Li, S.; Lan, W.; Hossen, M.A.; Qin, W.; Lee, K. Electrospun antibacterial and antiviral poly (ε-caprolactone)/zein/Ag bead-on-string membranes and its application in air filtration. Mater. Today Adv. 2021, 12, 100173.
- Borojeni, I.A.; Gajewski, G.; Riahi, R.A. Application of Electrospun Nonwoven Fibers in Air Filters. Fibers 2022, 10, 15.
- Ding, L.-G.; Wang, S.; Yao, B.-J.; Wu, W.-X.; Kan, J.-L.; Liu, Y.; Wu, J.; Dong, Y.-B. Covalent organic framework based multifunctional self-sanitizing face masks. J. Mater. Chem. A 2022, 10, 3346–3358.
- Zhang, W.; Guo, D.; Li, Z.; Shen, L.; Li, R.; Zhang, M.; Jiao, Y.; Xu, Y.; Lin, H. A new strategy to accelerate co-deposition of plant polyphenol and amine for fabrication of antibacterial nanofiltration membranes by in-situ grown Ag nanoparticles. Sep. Purif. Technol. 2022, 280, 119866.
- Murali, G.; Lee, M.; Modigunta, J.K.R.; Kang, B.; Kim, J.; Park, E.; Kang, H.; Lee, J.; Park, Y.H.; Park, S.Y. Ultraviolet–Ozone-Activation-Driven Ag Nanoparticles Grown on Plastic Substrates for Antibacterial Applications. ACS Appl. Nano Mater. 2022, 5, 8767–8774.
- Guo, C.; Cheng, F.; Liang, G.; Zhang, S.; Jia, Q.; He, L.; Duan, S.; Fu, Y.; Zhang, Z.; Du, M. Copper-based polymer-metal–organic framework embedded with Ag nanoparticles: Long-acting and intelligent antibacterial activity and accelerated wound healing. Chem. Eng. J. 2022, 435, 134915.
- Li, X.; Zhang, Y.; Kong, W.; Zhou, J.; Hou, T.; Zhang, X.; Zhou, L.; Sun, M.; Liu, S.; Yang, B. Cross-Linking of Centrifugally Spun Starch/Polyvinyl Alcohol (ST/PVA) Composite Ultrafine Fibers and Antibacterial Activity Loaded with Ag Nanoparticles. ACS Omega 2022, 7, 7706–7714.
- Ju, Y.; Han, T.; Yin, J.; Li, Q.; Chen, Z.; Wei, Z.; Zhang, Y.; Dong, L. Bumpy structured nanofibrous membrane as a highly efficient air filter with antibacterial and antiviral property. Sci. Total Environ. 2021, 777, 145768.
- Saud, Z.; Richards, C.A.; Williams, G.; Stanton, R.J. Anti-viral organic coatings for high touch surfaces based on smart-release, Cu2+ containing pigments. Prog. Org. Coat. 2022, 172, 107135.
- Raj, B.; Padhy, A.K.; Basu, S.; Mohapatra, M. Perspective of Organic-Based Antimicrobial Coating Materials: Implication Toward COVID-19. In COVID-19 Pandemic; Springer: Berlin/Heidelberg, Germany, 2022; pp. 75–89.
- Yu, Z.; Kadir, M.; Liu, Y.; Huang, J. Droplet-capturing coatings on environmental surfaces based on cosmetic ingredients. Chem 2021, 7, 2201–2211.
This entry is offline, you can click here to edit this entry!
