Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Antioxidants are prevalently used during rubber production to improve rubber performance, delay aging, and extend service life. Studies have revealed that their transformation products (TPs) could adversely affect environmental organisms and even lead to environmental events, which led to great public concern about environmental occurrence and potential impacts of rubber antioxidants and their TPs.
- rubber antioxidants
- transformation products
- toxic effects
1. Introduction
Many unidentified chemicals and related transformation products (TPs) are released into the environment during the life-cycle of commercial products. Efforts in evaluating their adverse impacts were usually not taken until these chemicals become globally ubiquitous. Rubber is a group of high-molecular-weight polymer materials with a property of elasticity at 20–27 °C [1], and rubber is one of such typical commercial products. Rubber products are mainly used in industrial and agricultural production, transportation, and national defense construction, and antioxidants are a group of the most important chemicals with widespread use in rubber products. Due to exposure to ozone (O3) (O3 oxidative degradation), light (photo-degradation), heat (thermo-degradation), redox processes, catalysis of heavy metals (e.g., copper) [2], radiation, and erosion of other chemicals and molds (bio-degradation), rubber products may become sticky, hard, brittle, or cracked after long-term use or storage [3]. O3 oxidative degradation is the most common pathway causing aging because of the strong oxidation effect of superoxide anion radical (O2•−) produced by O3. Rubber aging leads to a gradual reduction in its performance and even total loss of its use value, which paves the way for the addition of antioxidants in rubber. Antioxidants are added to natural rubber (NR) and synthetic rubber (SR) during mastication, which is the process of transforming rubber from a strong and elastic state to a soft and plastic state [4][5]. In addition, they could also be coated on the NR surface to achieve a similar effect [6]. In spite of this, the antioxidants may still be transformed in the environment due to oxidative degradation and may produce some novel compounds [7].
Given the global ubiquity of the antioxidants, the potential adverse impact of these chemicals and their TPs has gradually caused public concern. Many previous studies have noticed the degradation of antioxidants through the O3 oxidation, since the most observed TPs of antioxidants contains the quinone group and they showed high toxicity. Antioxidants and their TPs have been released into the environment, and may lead to adverse impacts on the local biota and even human health. Recently, it was reported that the rubber antioxidant N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD or antioxidant 4020), a typical tire rubber antioxidant, could enter the surrounding environment together with tire-wear particles (TWPs) [7][8]. Its TP (6PPD-quinone) could induce acute mortality in coho salmon, and therefore assumed to result in a large number of silver salmon deaths in the US Pacific Northwest [7]. In addition, large amounts of antioxidants used as rubber additives in tire filtrates have been observed to substantially reduce the survival and reproduction rates of soil worms by more than 25% and 50%, respectively [9]. This implies that rubber antioxidants in tire filtrates could disturb microorganisms in the surrounding soil, reduce the number of soil worms, and even threaten the terrestrial ecosystem by affecting soil organisms and their intestinal microbiota. These studies therefore aroused great attention on the environmental impacts of rubber antioxidants and their TPs.
2. Production and Use of Typical Rubber Antioxidants
Rubber antioxidants are defined as substances that could delay the aging of polymer compounds and prolong the service life of rubber products by inhibiting oxidation, heat, or light radiation [10]. To date, the annual global consumption of rubber antioxidants is over 700,000 tons, accounting for about 40% of the total amount of rubber additives. This is about twice higher than that of phosphorus flame retardants, a group of emerging pollutants which received great attention in the past decades [11]. China is one of the main countries producing rubber antioxidants, and the production accounts for more than 70% of the total amount globally. The production of rubber antioxidants in China ranged from 365,000 to 378,000 tons during 2016–2020, showing a constant annual trend [12]. Amine antioxidants are the main rubber antioxidants produced and used in China, of which 6PPD and 2,2,4-Trimethyl-1,2-dihydroquinoline (TMQ, RD) have the highest production, accounting for more than 80% of the total amine antioxidants. Moreover, the annual production of 6PPD is 189,500–208,600 tons, which accounts for about 55% of the total amount of amine antioxidants, followed by TMQ with an annual production of 102,800–126,000 tons (approximately 30% of the total amount) [13]. There is very little information about the exact production of the other types of rubber antioxidants in the world.
Antioxidants can be classified according to their antiaging mechanisms (counteracting oxygen, O3, and copper), effects on appearance (discoloration vs. non-discoloration, and contamination vs. non-contamination), specific function (heat resistance, bending, and crack resistance), and physicochemical properties (natural, physical, and chemical antioxidants). Natural antioxidants are only found in NR, such as amino acids, tocotrienol, and betaines [14], whereas physical and chemical antioxidants are widely used in various synthetic rubber products. The rubber-aging process comprises three stages: initiation, reaction, and termination [15][16], and the physical antioxidants are usually used to address the initiation stage of rubber aging. A film-isolating oxygen and O3 is formed on the surface of rubber products by directly applying or spraying, which can prevent the rubber from aging. By contrast, chemical antioxidants are usually used to address the reaction stage of rubber aging. According to the different fracture modes of the molecular chain of raw rubber materials, different chemical antioxidants are added to block the growth reaction chain during aging [15]. Chemical antioxidants are generally classified as amine, phenolic, heterocyclic, phosphite, and nickel salts (nickel dibutyl dithiocarbamate (NBC)) antioxidants according to their chemical structure (Figure 1). During the rubber production, various antioxidants are often used as a mixture to improve performance and ensure an antiaging effect.
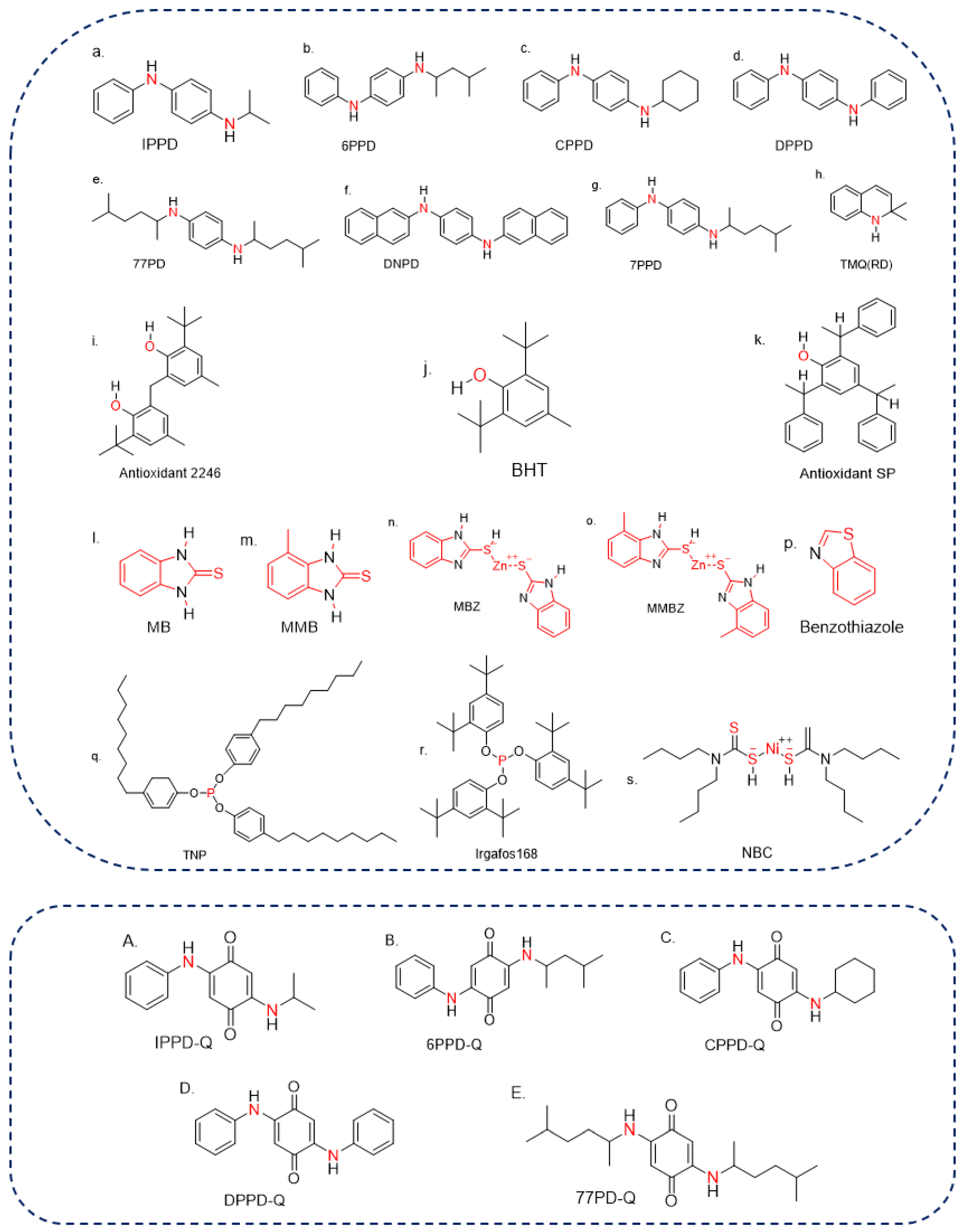
Figure 1. The structures of typical antioxidants and some of their TPs ((a–h) are for amine antioxidants, (i–k) are for phenolic antioxidants, (l–p) are for heterocyclic antioxidants, (q,r) are for phosphite antioxidants, (s) is for nickel salts antioxidants, (A–E) are for TPs, the red parts represent the similar structure of the same type of antioxidants).
2.1. Amine Antioxidants
Amine antioxidant is the most common rubber antioxidant, which was produced as early as the 1970s and widely used in the rubber industry. Typical amine antioxidants include diaryl-secondary amine, acetone-amine condensation product, p-phenylenediamine, and aldehyde-amine condensation product antioxidants [17]. The most common products include 6PPD, N,N′-bis(1,4-dimethylpentyl)-p-phenylenediamine (77PD), tris-(N-dimethylpentyl-p-phenylenediamine)-N,N′,N″-1,3,5-triazine (PPDTZ), 1,3,5-Triazine-2,4,6-triamine, N,N′,N″-tris [4-[(1,4-dimethylpentyl)amino]phenyl] (TMPPD), N-isopropyl-N′-phenylenediamine (IPPD), and the TMQ. The free radicals of an amine antioxidant could capture and combine with the active peroxide produced by the oxidation reaction of rubber molecular chain growth to form stable compounds, which could slow down the aging process [18]. Amine antioxidants show a great inhibitory effect on the aging process caused by oxygen and O3 oxidation, thermal interactions, buckling, and copper. However, they could cause photochromism under sunlight, resulting in the discoloration of white rubber. Thus, they are unsuitable for white and light-colored rubber products [19].
2.2. Phenolic Antioxidants
Phenolic antioxidants could be divided into alkylene phenolic, substituted monobasic phenolic, polybasic phenolic, and sulfurized disubstituted phenolic antioxidants [20]. Typical phenolic antioxidant products include 2,2′-methylenebis (6-tert-butyl-4-methyl-phenol) (antioxidant 2246), 2,6-di-tert-butyl-4-methylphenol (BHT (264)), and styrenated phenol (antioxidant SP). Among them, antioxidant 2246 has a good performance to protect rubber from aging caused by heat, oxygen, and metals. Because hydrogen in phenolic antioxidants can combine with the oxygen in air, their antiaging efficiency is therefore lowered compared with amine antioxidants [21][22]. By contrast, phenolic antioxidants have less effect on rubber color, and thus are widely used in light-colored rubber products [23].
2.3. Heterocyclic Antioxidants
Heterocyclic antioxidants are mainly used to prevent thermal and oxygen aging and could effectively prevent copper damage [24]. They are generally used in light-colored and transparent rubber products, as well as foam latex products. The commercial products of heterocyclic antioxidants mainly include 2-mercaptobenzimidazole (MB), 2-mercaptomethylbenzimidazole (MMB), 2-mercaptobenzimidazole zinc salt (MBZ), 2-mercaptomethylbenzimidazole zinc salt (MMBZ) and the benzothiazole derivatives [25]. Among them, MB and MBZ are the two most common products. MB is nontoxic but bitter, which makes it unsuitable for rubber products contacting with food, such as sanitary gloves and food container gaskets [26][27].
2.4. Phosphite Antioxidants
Phosphite, as a hydroperoxide-decomposing agent and a free-radical-trapping agent, plays a key role as an auxiliary antioxidant in polymer systems [28]. Phosphite antioxidants mainly include tris(nonylphenyl) phosphate (TNP), tris(1,2,2,6,6-pentamethylpiperidinyl) phosphite (GW-540), and tris(2,4-di-tert-butylphenyl) phosphite (Irgafos168). GW-540 is widely used in tires blended with styrene butadiene rubber and polybutadiene rubber. TNP is suitable for NR, SR, latex, and plastic products. As a stabilizer and antioxidant, it could endow rubber products with considerable heat resistance [29]. In addition, phosphite antioxidants have no influence on the color and luster of rubber. By the combined usage of phenols or amine antioxidants, they could effectively improve the comprehensive antiaging abilities of rubber [30].
This entry is adapted from the peer-reviewed paper 10.3390/ijerph192114595
References
- ASTM D1566-21a; Standard Terminology Relating to Rubber. ASTM International: West Conshohocken, PA, USA, 2020.
- Pan, Z.D.; Ding, Y.J.; Yan, L.; Li, X.M.; Jiao, G.P.; Luo, H.Y. Study on copper-based catalysts for synthesis of N,N’-bis(1,4-dimethylpentyl)-p-phenylenediamine from reductive alkylation of p-Nitroaniline with 5-methyl-2-hexanone. Catal. Lett. 2008, 122, 115–120.
- Sarkar, P.; Bhowmick, A.K. Sustainable rubbers and rubber additives. J. Appl. Polym. Sci. 2017, 135, 45701.
- Öncel, Ş.; Kurtoğlu, B.; Karaağaç, B. An alternative antioxidant for sulfur-vulcanized natural rubber. J. Elastomers Plast. 2018, 51, 440–456.
- Pike, M.; Watson, W.F. Mastication of rubber mechanism of plasticizing by cold mastication. J. Polym. Sci. 2010, 9, 229–251.
- Maher, B.M.; Rezaali, J.; Jalili, K.; Abbasi, F. Effects of various treatments on silicone rubber surface. Rubber Chem. Technol. 2017, 90, 108–125.
- Tian, Z.Y.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R. A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science 2020, 371, 185–189.
- Men, Z.Y.; Zhang, X.F.; Peng, J.F.; Zhang, J.; Fang, T.G.; Guo, Q.Y.; Wei, N.; Zhang, Q.J.; Wang, T.; Wu, L.; et al. Determining factors and parameterization of brake wear particle emission. J. Hazard. Mater. 2022, 434, 128856.
- Ding, J.; Zhu, D.; Wang, H.T.; Lassen, S.B.; Zhu, Y.G. Dysbiosis in the gut microbiota of soil fauna explains the toxicity of tire tread particles. Environ. Sci. Technol. 2020, 54, 7450–7460.
- Guo, X.H.; Luo, Y.F.; Chen, L.J.; Zhang, B.W.; Chen, Y.J.; Jia, D.M. Biomass antioxidant silica supported tea polyphenols with green and high-efficiency free radical capturing activity for rubber composites. Compos. Sci. Technol. 2022, 220, 109290.
- Zhang, X. Analysis of the Current Market Situation of Phosphorous Flame Retardants Industry in China in 2020 Shows Significant Market and Performance Advantages. Available online: https://www.huaon.com/channel/trend/741225.html (accessed on 20 April 2022).
- Dai, M.X.; Liang, C. Current situation and market analysis of rubber antioxidant industry. Econ. Anal. China Pet. Chem. Ind. 2014, 7, 3.
- Association, C.R.I. Statistical Analysis of China’s Rubber Additives Industry in 2020. Available online: http://www.rubberchem.com.cn/info/202132/20213295208.shtml (accessed on 27 January 2022).
- Abad, L.V.; Relleve, L.S.; Aranilla, C.T.; Aliganga, A.K.; San Diego, C.M.; dela Rosa, A.M. Natural antioxidants for radiation vulcanization of natural rubber latex. Polym. Degrad. Stab. 2002, 76, 275–279.
- Zheng, T.; Zheng, X.; Zhan, S.; Zhou, J.; Liao, S. Study on the ozone aging mechanism of natural rubber. Polym. Degrad. Stab. 2021, 186, 109514.
- Li, G.-Y.; Koenig, J.L. A review of rubber oxidation. Rubber Chem. Technol. 2005, 78, 355–390.
- Lu, N.; Shen, M.; Hou, Z.; Prakashan, K.; Xin, Z. Effectiveness of different kinds of antioxidants in resin-cured bromobutyl rubber vulcanizates. Adv. Polym. Technol. 2017, 37, 2075–2084.
- Bravar, M.; Rolich, J.; Biga, N. Protection of natural rubber films against thermal ageing by addition of amine and phenolic anti-oxidants. Eur. Polym. J. 1980, 16, 637–640.
- Sahakaro, K.; Naskar, N.; Datta, R.N.; Noordermeer, J.W.M. Reactive blending, reinforcement and curing of NR/BR/EPDM compounds for tire sidewall applications. Rubber Chem. Technol. 2007, 80, 115–138.
- Zeb, A. Applications of phenolic antioxidants. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021; pp. 385–411.
- Ahmadi, S.; Arabi, H. Enhanced thermo-oxidative stability through covalent attachment of hindered phenolic antioxidant on surface functionalized polypropylene. Polymer 2018, 138, 41–48.
- Barret, J.; Gijsman, P.; Swagten, J.; Lange, R. The interaction of a phenolic anti-oxidant and an aromatic amine in a thermo-oxidative ageing process. Polym. Degrad. Stab. 2002, 75, 367–374.
- Sulekha, P.; Joseph, R.B. Preparation and characterisation of novel polymer bound phenolic antioxidants and its use in natural rubber. J. Elastomers Plast. 2003, 35, 85–97.
- Herdan, J.M.; Giurginca, M. Grafting antioxidants phenols with mercaptoheterocyclic substituents as antioxidants for dienic rubbers. Polym. Degrad. Stab. 1993, 41, 157–162.
- Yakout, E.M.A.; El-Sabbagh, S.H. New uracil derivatives as antioxidants for natural rubber. Pigment Resin Technol. 2007, 36, 224–234.
- Hu, D.C.; Jia, Z.X.; Zhong, B.C.; Chen, Y.J.; Luo, Y.F. A facile and green preparation of nanosilica-supported antioxidant and its reinforcement and antioxidation effect on styrene-butadiene rubber. Int. J. Polym. Anal. Charact. 2016, 21, 185–197.
- Wu, Y.P.; Zhang, Z.Z.; Wei, L.Q.; Yang, S.Z. A combined experimental and molecular simulation study of factors influencing the selection of antioxidants in butadiene rubber. J. Phys. Chem. B Condens. Matter Mater. Surf. Interfaces Biophys. 2017, 121, 1413–1425.
- Humphris, K.J.; Scott, G. Mechanisms of antioxidant action. Pure Appl. Chem. 1973, 36, 163–176.
- Ismail, M.N.; Yehia, A.A.; Korium, A.A. Evaluation of some arylphosphites as antioxidants and antifatigue agents in natural rubber and styrene–butadiene rubber vulcanizates. Polym. Degrad. Stab. 2001, 74, 247–253.
- Basfar, A.A.; Abdel-Azizl, M.M.; Mofti, S. Stabilization of γ-radiation vulcanized EPDM rubber against accelerated aging. Polym. Degrad. Stab. 1999, 66, 191–197.
This entry is offline, you can click here to edit this entry!
