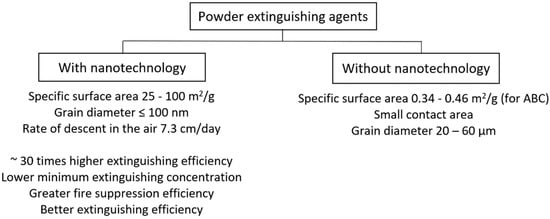
A typical extinguishing powder available on the market (e.g., ABC extinguishing powder: specific surface 0.34–0.46 m2/g) may prove insufficient due to the sedimentation time and small contact surface, e.g., when extinguishing a gas flame. The solution for extinguishing this type of fire are nanopowders (grain diameter approx. 100 nm, specific surface area 25–100 m2/g), which fall in the air at a speed of 7.3 cm/day, creating an aerosol cloud, thanks to which the extinguishing efficiency is approx. 30 times greater than common powder.
- extinguishing agents
- nanotechnology
- application
1. Introduction—Extinguishing Agents
- –
-
Class A—the presence of solids, including materials such as paper, wood and plastic.
- –
-
Class B—the presence of liquids, such as paraffin, petrol and oil.
- –
-
Class C—flammable gases, such as propane, butane and methane.
- –
-
Class D—the presence of metal products, such as aluminum, magnesium and titanium.
- –
-
Class E—caused by electricity or ones that involve electrical equipment and apparatus.
- –
-
Class F—most commonly occur in kitchens and food preparation facilities, and involve cooking oil or fat [7].

2. Powder Extinguishing Agents
| Extinguishing Powder Base | Chemical Formula | Class of Fires Being Extinguished |
|---|---|---|
| Potassium hydrogen carbonate | KHCO3 | BC |
| Urea + Potassium hydrogen carbonate | NH2CONH2 + KHCO3 | BC |
| Sodium hydrogen carbonate | NaHCO3 | BC |
| Sodium tetraborate | Na2B4O7 · 10H2O | D |
| Potassium chloride | KCl | D |
| Sodium chloride | NaCl | D |
- –
-
from 60 to 80% by weight of the total powder in the range of 20 ÷ 60 μm;
- –
-
from 10 to 15% by weight of the total powder in the range of 100 ÷ 200 μm [20].
| Nanoparticles | Characteristic | Effect | Ref. |
|---|---|---|---|
| NaHCO3 | NP-NaHCO3 on porous zeolite | improving fire suppression efficiency | [21][25] |
| Ferrocen | sized nanoparticles < 100 nm | greater efficiency of extinguishing flames and stopping fires | [28] |
| Mn, Zn | sized nanoparticles < 100 nm | greater efficiency of extinguishing flames and stopping fires | [29] |
| Mg(OH)2 | NP-Mg(OH)2 with the addition of melamine cyanurate and phosphorus ODOPB—10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide | higher extinguishing efficiency, shorter fire extinguishing time by about 45%, lower consumption of extinguishing powder | [24] |
| SiO2 | hydrophobized nanoparticles with a size of about 65 nm as an additive to extinguishing powders based on ammonium phosphates | improving the fluidity of powders | [40] |
| SiO2 | mesoporous nanoparticles of various structures (MCM-41, MCM-48 and SBA-15), characterized by a large specific surface (>1400 m2/g) | improving the fluidity of powders | [41] |
- –
-
changing the composition of the extinguishing powder particles after incorporation of nanoparticles, which modifies the powder’s mechanism of action and enables the extinguishing efficiency of such powder to be increased;
- –
-
immobilization of nanoparticles on the surface of extinguishing powder particles, which changes their physical properties, such as fluidity or hydrophobicity, significantly improving the extinguishing efficiency of the powder.
3. Foam Concentrates
Foam concentrates used in rescue and firefighting operations are used to generate foam, i.e., bubbles made of liquid. The EN 1568 series of standards specifies the requirements for the physicochemical properties and minimum effectiveness of extinguishing foams intended for the production of medium-, light- and heavy-expansion foams suitable for surface application to liquids immiscible and/or miscible with water. The EN 1568 standard [51] distinguishes types of foam extinguishing agents such as: synthetic (S), protein (P), fluoroprotein (FP), alcohol-resistant (AR), aqueous film-forming concentrates (AFFF), fluoroprotein water film-forming agents (FFFP) and fluorine free foam concentrates (F3).
The analysis of the results of works the application of nanotechnology for the modernization of foaming agents showed that silica nanoparticles are most often used in the works (Table 3).
Table 3. Possibilities of using metal nanoparticles and their compounds in extinguishing foams.
|
Nanoparticles |
Characteristic |
Effect |
Ref. |
|
TiO2 |
H-TiO2/gel system with a three-dimensional network structure |
flame retardant effect, longer ignition time |
[60] |
|
Al(OH)3 |
addition to the composite foaming agent solution, mass concentration 2% |
high foam stability, good flame retardant and smoke suppression properties |
[62] |
|
SiO2, Al(OH)3, Al2O3, Mg(OH)2, MgO, FeO, Sb2O3 |
evenly mixed with the complex surfactant solution |
improving the thermal insulation properties of the foam layer, affects the efficiency of extinguishing and controlling solid and liquid fires |
[64] |
|
SiO2 |
ordered nanoparticles with a narrow particle size distribution of ∼10−20 nm |
50-times higher extinguishing efficiency, biodegradation index—3 days |
[68] |
|
SiO2 |
SiO2-TTAB mixture, with an NP-SiO2 concentration of 2 wt%. |
strong foam stabilizing effect |
[69] |
|
SiO2 |
NP-SiO2 modified with chloro- and methyl- silanes |
stabilization of the foam even for several days without disproportionation |
[70] |
|
Mex(CO3)y |
surfactants in the form of nanoencapsulated metal carbonate ions (NEIL) |
formation of a stable foam and the release of CO2 |
[73,74] |
4. Other Fire Extinguishing Agents
The most frequently used extinguishing agent, mainly due to its availability, low price and relatively good extinguishing properties, is water. The extinguishing property of water is related to its cooling effect. It lowers the temperature of the burning material and the combustion zone. The water vapor generated during the extinguishing inhibits the reactions of free radicals with flammable gases, thanks to which it dilutes the combustion zone and has an insulating effect [75].
For example, Dali et al. [78] showed that the quenching time of liquid hydrocarbons by suspensions containing carbon nanostructures (CNS), such as functionalized multi-walled carbon nanotubes (MWCNTs), is on average 3.5–5.0 times shorter than the quenching time of liquids with finely divided water. Wetting agents and additives increase the intensity of the heat sink, creating a film on the surface of the burning oil product. Carbon nanotubes increase the thermal conductivity and change the rheological properties of liquids at low concentrations [79]. It has also been found that the CNS water-based slurries are extinguishing agents with a predominantly cooling and diluting effect. Suspension droplets enter the combustion zone, which causes intense heating to the boiling point. Such a process causes evaporation and cooling of the combustion zone, and the flame is extinguished with a sufficient amount of water vapor in the combustion zone [78,80]. However, it should be remembered that too high a concentration of nanoparticles may lead to aggregation of nanoparticles, which reduces the effective thermal conductivity of the suspensions and the specific heat of vaporization.
An interesting solution is the invention [91], which allows the use of nanocrystalline particles with a relatively large surface area to reduce the amount of various substances generated during fires and to suppress the fire itself. The results of the research showed that nanocrystalline particles can come from the group consisting of metal oxides, metal hydroxides, carbonates, bicarbonates, phosphorus, inorganic phosphorus compounds, boron compounds, antimony compounds, molybdenum compounds, titanium compounds, zirconium compounds, zinc compounds, amidosulfonates, sulfates, bromine compounds, chlorine compounds and mixtures thereof. Metal oxides and metal hydroxides Mg, Sr, Ba, Ca, Ti, Zr, Fe, V, Mn, Ni, Cu, Al, Si, Zn, Ag, Mo, Sb and mixtures thereof are the most preferred nanocrystalline materials. However, sodium, aluminum, magnesium and calcium hydroxides, carbonates and bicarbonates are most preferred. It has been shown that the size of the nanocrystalline particles should be less than 25 nm; nonetheless, the most optimal particles are about 1–20 nm, especially between about 2 and 10 nm. In contrast, the values characterizing the multipoint Brunauer–Emmett–Teller (BET) surface area should be at least about 15 m2/g, with the most optimal being at least about 70 m2/g and most preferably in the range of about 200–850 m2/g [91].
The effectiveness of smoke removal is determined by the amount of nanocrystalline particles dispensed in a given area (i.e., mass concentration of nanoparticles), aerodynamic geometric mean diameter (GMD) of the particles and the settling velocity of the particles. The most optimal amount of nanocrystalline particles to be dispensed in a region is between about 1–5 g/m3. The nanocrystalline particles at first must be scattered in the area affected by the smoke to absorb some of the smoke, especially the carbon particles, which tend to obscure visibility. The developed solution also allows a reduction of the amount of various toxic compounds, such as acrolein, toluene diisocyanate, formaldehyde, isocyanates, HCN, CO, NO, HF and HCl generated during a fire [91].
Nanotechnology creates opportunities to improve the effectiveness of firefighting (Table 4) and thus the safety of users and people involved in the event.
Table 4. Possibilities of using nanotechnology to improve the efficiency of firefighting.
|
Nanoparticles |
Characteristic |
Effect |
Ref. |
|
CNS |
suspension of carbon nanostructures (CNS) in the form of functionalized multi-walled carbon nanotubes (MWCNT) |
shorter fire extinguishing time, mainly cooling and diluting effect |
[78] |
|
diatomite, kaolin, bentonite, attapulgite, SiO2 |
inorganic nanoparticles added to a special superabsorbent resin |
improving the adhesion of hydrogel extinguishing agent, better extinguishing effect |
[81] |
|
mist of H2O |
Water Mist technology |
reducing the amount of water needed to extinguish the fire, the ability to avoid obstacles, quickly lowering the temperature |
[82,83] |
|
ultrasonic fragmentation of H2O particles |
[85] |
||
|
system containing dimethyl methylphosphonate (DMMP) |
reducing the concentration of toxic and combustible gases and coal dust, the thermal barrier effectively reduces the temperature of the flame |
[84] |
|
|
water glass |
nano fiberglass |
extinguishing all types of fires (including fires of lubricants, liquids and gases) |
[90] |
|
hydroxides, carbonates and bicarbonates of Na, Al, Mg and Ca |
nanocrystalline particles with a size of about 2-10 nm, with a surface area of 200–850 m2/g |
reduction in part of the smoke (especially carbon particles) and the amount of various toxic compounds formed during a fire |
[91] |
The use of nanoparticles shortens the fire extinguishing time, reduces the concentration of toxic and combustible gases and reduces the amount of dust, which significantly reduces pollution and migration of pollutants along with smoke over longer distances. It should also be noted that the fragmentation of water particles to sizes < 100 nm allows a reduction of the amount of water needed to extinguish the fire and the amount of fire extinguishing sewage.
5. Conclusions
The fire protection system must be constantly improved as a consequence of changes taking place in the economic, social and legal space. In addition to preventive measures, research is necessary in the field of tools used in rescue operations. Solutions based on nanotechnology are being introduced more and more often. They are aimed at improving the key properties of individual extinguishing agents and thus their effectiveness in a firefighting situation. In this context, the requirements for efficiency improvement , which are becoming more and more demanding.
The analysis of the type of nanoparticles used in the processes of modification of extinguishing agents shows that inorganic nanoparticles, mainly silica and oxides, hydroxides and bicarbonates/carbonates of alkali metals and aluminum, are the most widely used. The introduction of these nanoparticles to standardly used powders or foaming agents allows for faster fire extinguishing, greater efficiency, reduction in the emission of toxins into the environment and reduction in heat emission to the environment. An interesting solution is also the reduction of water particles to the size of nanoparticles, using, among others, ultrasound, which creates a thermal barrier and reduces the time of extinguishing the fire, as well as reduces the consumption of the extinguishing agent. Thus, it improves the safety of people involved in the event and allows you to reduce the costs of occurring fires.
However, selection of nanoparticles must be appropriate, because at higher concentrations they may be subject to aggregation, which in turn reduces the effectiveness of the quenching process. At the same time, it should be noted that foams and extinguishing powders may have a negative impact on the environment due to the use of various types of chemical compounds with greater or lesser chemical activity in their production. As a result of the application of such measures, some areas can become contaminated and require further treatment, consisting in the neutralization of substances created during the fire and extinguishing, at least. Therefore, in order to further improve the extinguishing efficiency and the resistance of the extinguishing agent, as well as to minimize environmental pollution, it is necessary to develop extinguishing agents that can extinguish various complex fires and, at the same time, remain stable and environmentally friendly.
Solutions implemented on the basis of nanotechnology contribute to faster cooling of the environment, improved visibility in case of high smoke and more effective fire extinguishing action. However, work carried out in this scope and the modifications introduced need to consider the toxicity of nanoparticles, the possibility of their migration and the negative impact both on the environment and on humans, including those involved in the incident.
This entry is adapted from the peer-reviewed paper 10.3390/ma15248876
References
- Tępiński, J.; Klapsa, W.; Cygańczuk, K.; Lesiak, P.; Lewak, M.W. Testing of Large Scale Pool Fire of Technical Ethanol. SFT 2022, 59, 96–109, https://doi.org/10.12845/sft.59.1.2022.5.
- Yilmaz-Atay, H.; Wilk-Jakubowski, J.Ł. A Review of Environmentally Friendly Approaches in Fire Extinguishing: From Chemical Sciences to Innovations in Electrical Engineering. Polymers 2022, 14, 1224. https://doi.org/10.3390/polym14061224.
- Tidey, A. Fighting Europe’s Fires: Inside the EU’s Emergency Response Centre. Available online: https://www.euronews.com/my-europe/2022/07/25/fighting-europes-fires-inside-the-eus-emergency-response-centre (ac-cessed on 6 September 2022).
- Statistics Poland. Available online: https://stat.gov.pl/en/search/search.html?query=fire (accessed on 23 August 2022).
- Antonov, A.; Skorobagatko, T.; Yakovchuk, R.; Sviatkevych, O. Interaction of Fire-Extinguishing Agents with Flame of Diesel Bio Fuel and Its Mixtures, Sci. Pap. Main Sch. Fire Serv. 2020, 2020, 73. https://doi.org/10.5604/01.3001.0014.0763.
- Park, C. Fire Extinguishers. 5 Types of Fire Extinguishers: A Guide to Using the Right Class. IFSEC GLOBAL. Available online: https://www.ifsecglobal.com/global/choose-right-type-fire-extinguisher/ (accessed on 6 September 2022).
- City Fire Protection in London. Available online: https://www.cityfire.co.uk/news/fire-extinguishers-types/ (accessed on 6 September 2022).
- EN 615:2009; Fire Protection—Fire Extinguishing Media—Specifications for Powders (Other Than Class D Powders). ISS Z021. European Norm: 2009. Newark, DE 19702. United States
- ISO 7202:2018; Fire Protection—Fire Extinguishing Media—Powder. Technical Committee: ISO/TC 21/SC 6 Foam and Powder Media and Firefighting Systems Using Foam and Powder. ICS 13.220.10 Fire-Fighting. ISO: 2018. Geneva, Switzerland
- Izak, P.; Biel, M.; Mastalska-Popławska, J.; Janik, P.; Mortka, P.; Lesiak, P. The Effect of Magnesium Hydroxide Addition on the Extinguishing Efficiency of Sodium Bicarbonate Powders. Materials 2022, 15, 3449. https://doi.org/10.3390/ma15103449.
- Zhang, X.; Ismail, M.H.S.; Ahmadun, F.-R.; Abdullah, N.; Hee, C. Hot Aerosol Fire Extinguishing Agents and The Associated Technologies: A Review. Braz. J. Chem. Eng. 2015, 32. https://doi.org/10.1590/0104-6632.20150323s00003510.
- Li, H.; Fenf, L.; Du, D.; Guo, X.; Hua, M.; Pan, X. Fire suppression performance of a new type of composite superfine dry powder. Fire Mater. 2019, 43, 905–916.
- Li, Y.; Qi, L.; Liu, Y.; Qiao, J.; Wang, M.; Liu, X.; Li, S. Recent Advances in Halogen-Free Flame Retardants for Polyolefin Cable Sheath Materials. Polymers 2022, 14, 2876. https://doi.org/10.3390/polym14142876.
- Fatsa Fire, Most Effective Fire Extinguishing Methods. 2022. Available online: https://www.fatsafire.com/most-effective-fire-extinguishing-methods/ (accessed on 26 September 2022).
- Izak, P.; Kidoń, A.; Mastalska-Popławska, J. Mechanism of Fire-extinguishing Aerosol’s Action. BiTP 2017, 46, 56–71. https://doi.org/10.12845/bitp.46.2.2017.4.
- Wilczkowski, S. Inhibition effect of chemical compounds in selected extinguishing agents. Saf. Fire Technol. 2010, 19, 99–105.
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting-How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. https://doi.org/10.1002/anie.201711735 .
- Li, H.; Hua, M.; Pan, X.; Li, S.; Guo, X.; Zhang, H.; Jiang, J. The reaction pathway analysis of phosphoric acid with the active radicals: A new insight of the fire-extinguishing mechanism of ABC dry powder. J. Mol. Model. 2019, 25, 255. https://doi.org/10.1007/s00894-019-4136-y.
- Wang, Q.; Wang, F.; Li, C.; Li, Z.; Li, R. Fire extinguishing performance and mechanism for several typical dry water extin-guishing agents. RSC Adv. 2021, 11, 9827–9836. https://doi.org/10.1039/d1ra00253h.
- Rakowska, J.; Radwan, K.; Ślosorz, Z. Comparative Study of the Results of the Extinguishing Powder Grain Size Analysis Carried out by Different Methods. BiTP 2014, 34, 57–64. https://doi.org/10.12845/bitp.34.2.2014.5.
- Ni, X.; Kuang, K.; Yang, D.; Jin, X.; Liao, G. A new type of fire suppressant powder of NaHCO3/zeolite nanocomposites with core–shell structure. Fire Saf. J. 2009, 44, 968–975. https://doi.org/10.1016/j.firesaf.2009.06.004.
- Kuang, K.; Chow, W.K.; Ni, X.; Yang, D.; Zeng, W.; Liao, G. Fire suppressing performance of superfine potassium bicarbonate powder. Fire Mater. 2011, 35, 353–366 https://doi.org/10.1002/fam.1058.
- Haiqiang, L.; Ruowen, Z.; Jiaxin, G.; Siuming, L.; Yuan, H. Good dry powder to suppress high building fires. APCBEE Procedia 2014, 9, 291–295. https://doi.org/10.1016/j.apcbee.2014.01.052.
- Ibrahim, H.; Patruni, J.R. Experimental investigation on extinguishing performance of a novel nanocomposite for gaseous fires. J. Loss Prev. Process Ind. 2020, 65, 104143 https://doi.org/10.1016/j.jlp.2020.104143.
- Ni, X.; Kuang, K.; Wang, X.; Liao, G. A New Type of BTP/Zeolites Nanocomposites as Mixed-phase Fire Suppressant: Preparation, Characterization, and Extinguishing Mechanism Discussion. Journal of Fire Sciences 2009, 28(1), 5–25. doi:10.1177/0734904109340763
- Du, D.; Pan, X.; Hua, M. Experimental Study on Fire Extinguishing Properties of Compound Superfine Powder. Procedia Eng. 2018, 211, 142–148. https://doi.org/10.1016/j.proeng.2017.12.126.
- Kaiqian, K.; Xin, H.; Guangxuan, L. A comparison between superfine magnesium hydroxide powders and commercial dry powders on fire suppression effectiveness. Process Saf. Environ. Prot. 2008, 86, 182–188. https://doi.org/10.1016/j.psep.2007.11.002.
- Koshiba, Y.; Takahashi, Y.; Ohtani, H. Flame Suppression Ability of Metallocene (nickelocene, cobaltocene, ferrocene, man-ganocene, and chromocene). Fire Saf. J. 2012, 51, 10–17. https://doi.org/10.1016/j.firesaf.2012.02.008.
- Linteris, G.T.; Knyazev, V.D.; Babushok, V.I. Inhibition of Premixed Methane Flames by Manganese and Tin Compounds. Combust. Flame 2002, 129, 221–238. https://doi.org/10.1016/S0010-218000346-2.
- Łukaszczuk, P. The Application of Nanotechnology in Fire Protection. BiTP 2016, 42, 95–102. doi: 10.12845/bitp.42.2.2016.9.
- Rabajczyk, A.; Zielecka, M.; Popielarczyk, T.; Sowa, T. Nanotechnology in Fire Protection—Application and Requirements. Materials 2021, 12, 7849. doi: 10.3390/ma14247849.
- Biel, M.; Izak, P.; Skubacz, K.; Stempkowska, A.; Mastalska-Popławska, J. The Effect of Humidity on the Atomization Process and Structure of Nanopowder Designed for Extinguishment. Materials 2021, 14, 3329. https://doi.org/10.3390/ma14123329.
- Rabajczyk. A.; Zielecka, M.; Porowski, R.; Hopke, P.K. Metal nanoparticles in the air: State of the art and future perspectives. Environ. Sci. Nano 2020, 7, 3233. https://doi.org/10.1039/d0en00536c.
- Kunin, A.V.; Smirnov, S.A.; Lapshin, D.N.; Semenov, A.D.; Il’in, A.P. Technology development for the production of ABCE fire extinguishing dry powders. Russ. J. Gen. Chem. 2016, 86, 450−459. https://doi.org/10.1134/S1070363216020456.
- CEN/TR 15276-1 Fixed firefighting systems—Condensed aerosol extinguishing systems—part. 1: Requirements and test methods for components. EUROPEAN COMMITTEE FOR STANDARDIZATION, Management Centre: rue de Stassart, 36 B-1050 Brussels. 2009.
- Xie, J.; Zhang, J.; Ding, C.; Wang, X. Hydrophobic nano SiO2 as flow-enhancing additives and flame retardant synergizes with CaCO3 to suppress gas explosion. RSC Adv. 2021, 11, 4672–4681. DOI: 10.1039/D0RA09223A.
- Qu, L.; Morton, D.A.V.; Zhou, Q. Particle engineering via mechanical dry coating in the design of pharmaceutical solid dosage forms. Curr. Pharm. Des. 2015, 21, 5802−5814. https://doi.org/10.2174/1381612821666151008151001.
- Zimmerman, I.; Eber, M.; Meyer, K. Nanomaterials as flow regulators in dry powders. Z. Phys. Chem. 2004, 218, 51−102. https://doi.org/10.1524/zpch.218.1.51.25388.
- Hare, C.; Zafar, U.; Ghadiri, M.; Freeman, T.; Clayton, J.; Murtagh, M.J. Analysis of the dynamics of the FT4 Powder Rheom-eter. Powder Technol. 2015, 285, 123−127. https://doi.org/10.1016/j.powtec.2015.04.039.
- Shamsutdinov, A.S.; Kondrashova, N.B.; Valtsifer, I.V.; Bormashenko, E.; Huo, Y.; Saenko, E.V.; Pyankova, A.V.; Valtsifer, V.A. Manufacturing, Properties, and Application of Nanosized Superhydrophobic Spherical Silicon Dioxide Particles as a Func-tional Additive to Fire Extinguishing Powders. Ind. Eng. Chem. Res. 2021, 60, 11905–11914. https://doi.org/10.1021/acs.iecr.1c01999.
- Saenko, E.V.; Huo, Y.; Shamsutdinov, A.S.; Kondrashova, N.B.; Valtsifer, I.V.; Valtsifer, V.A. Mesoporous Hydrophobic Silica Nanoparticles as Flow-Enhancing Additives for Fire and Explosion Suppression Formulations. ACS Appl. Nano Mater. 2020, 3, 2221–2233 https://dx.doi.org/10.1021/acsanm.9b02309.
- Foster, J.; Doll, J. Particle size effect on talc lubricant activity. In American Association of Pharmaceutical Scientists Annual Meeting Poster Session; American Association of Pharmaceutical Scientists. Specialty Minerals Inc. 2004; Easton PA, USA. p. 18.
- Yang, J.; Sliva, A.; Banerjee, A.; Dave, R.N.; Pfeffer, R. Dry particle coating or improving the flowability of cohesive powders. Powder Technol. 2005, 158, 21−33. https://doi.org/10.1016/j.powtec.2005.04.032.
- Zegzulka, J.; Jezerska, L.; Zadrapa, F.; Zidek, M.; Gelnar, D. Flow characterization methods of glidants. In Proceedings of the 8th International Conference on Nanomaterials-Research & Application, Brno, Czech Republic, 19−21 October 2016.
- Veregin, R.P.N.; Bartha, R. A quantitative cohesive force mechanism for powder flow: The role of metal oxide surface addi-tives in xerographict oner. J. Imaging Sci. Technol. 2001, 45, 571−578.
- Reding, N.S.; Shiflett, M.B. Characterization of Thermal Stability and Heat Absorption for Suppressant Agent/Combustible Dust Mixtures via Thermogravimetric Analysis/Differential Scanning Calorimetry. Ind. Eng. Chem. Res. 2019, 58, 4674−4687. https://doi.org/10.1021/acs.iecr.8b06143.
- Park, E.J.; Cho, Y.K.; Kim, D.H.; Jeong, M.-G.; Kim, Y.H.; Kim, Y.D. Hydrophobic Polydimethylsiloxane (PDMS) Coating of Mesoporous Silica and Its Use as a Preconcentrating Agent of Gas Analytes. Langmuir 2014, 30, 10256−10262. https://doi.org/10.1021/la502915r.
- Jonat, S. Mechanism of Hydrophilic and Hydrophobic Colloidal Silicon Dioxide Types as Glidants. PhD Dissertation, Uni-versität Tübingen, Tübingen, Germany, April 2005.
- Kojima, T.; Elliott, J.A. Incipient flow properties of two component fine powder systems and their relationships with bulk density and particle contacts. Powder Technol. 2012, 228, 359−370. https://doi.org/10.1016/j.powtec.2012.05.052.
- Kojima, T.; Elliott, J.A. Effect of silica nanoparticles on the bulk flow properties of fine cohesive powders. Chem. Eng. Sci. 2013, 101, 315−328. https://doi.org/10.1016/j.ces.2013.06.056.
- EN 1568-1:2018; Fire Extinguishing Media—Foam Concentrates—Part 1: Specification for Medium Expansion Foam Concen-trates for Surface Application to Water-Immiscible Liquids. EUROPEAN COMMITTEE FOR STANDARDIZATION, Man-agement Centre: Rue de la Science 23, B-1040 Brussels 2018.
- Roth, J.; Abusallout, I.; Hill, T.; Holton, C.; Thapa, U.; Hanigan, D. Release of Volatile Per- and Polyfluoroalkyl Substances from Aqueous Film-Forming Foam. Environ. Sci. Technol. Lett. 2020, 7, 164–170. https://doi.org/10.1021/acs.estlett.0c00052.
- Jiang, N.; Sheng, Y.; Li, C.; Lu, S. Surface activity, foam properties and aggregation behavior of mixtures of short-chain fluo-rocarbon and hydrocarbon surfactants. J. Mol. Liq. 2018, 268, 249–255. https://doi.org/10.1016/j.molliq.2018.07.055.
- Wendong Kang, W.; Yan, L.; Ding, F.; Guo, X.; Xu, Z. Experimental study on fire-extinguishing efficiency of protein foam in diesel pool fire. Case Stud. Therm. Eng. 2019, 16, 100557. https://doi.org/10.1016/j.csite.2019.100557.
- Commission Regulation (EU) 2017/1000 of 13 June 2017 Amending Annex XVII to Regulation (EC) No 1907/2006 of the Eu-ropean Parliament and of the Council on Registration, Evaluation, Authorization and Use of Chemical Restrictions (REACH) for Perfluorooctanoic Acid (PFOA), Its Salts and Derivatives. Available online: https://www.legislation.gov.uk/eur/2017/1000/annex?view=plain (accessed on: 5 October 2022).
- Kuti, R. Advantages of Water Fog Use as a Fire Extinguisher. AARMS 2015, 14, 259–264. https://doi.org/10.32565/aarms.2015.2.11.
- Denkov, N.; Tcholakova, S.; Politova-Brinkova, N. Physicochemical control of foam properties. Curr. Opin. Colloid Interface Sci. 2020, 50, 101376. https://doi.org/10.1016/j.cocis.2020.08.001.
- Dlugogorski, B.Z.; Kennedy, E.M.; Schaefer, T.H.; Vitali, J.A. What Properties Matter In Fire-Fighting Foams? Whitepaper. In Proceedings of the National Research Institute of Fire and Disaster 2nd NRIFD Symposium—Conference Proceedings, Tokyo, Japan, July 17-19, 2002. Available online: https://www.kappetijn.eu/wp-content/uploads/2019/05/Solberg-what-properties-matther-in-foam.pdf (accessed on: 5 October 2022).
- Fan, X.; Guan, X.; Zhang, M.; Liu, Y.; Li, Y. Aqueous foam synergistically stabilized by the composite of lignin nanoparticles and surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128727. https://doi.org/10.1016/j.colsurfa.2022.128727.
- Li, Y.; Xiao, G.; Li, F.; Guo, Y.; Chen, C.; Chen, C.; Li, R.; Yang, Z. A novel H-TiO2/gel co-stabilized three-dimensional network synergistic fire-retardant foam gel for coal-pile. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129642. https://doi.org/10.1016/j.colsurfa.2022.129642.
- Sheng, Y.; Peng, Y.; Zhang, S.; Guo, Y.; Ma, L.; Wang, Q.; Zhang, H. Study on Thermal Stability of Gel Foam Co-Stabilized by Hydrophilic Silica Nanoparticles and Surfactants. Gels 2022, 8, 123. https://doi.org/10.3390/gels8020123.
- Zhang, L.; Yunpeng Bian, Y.; Kuai, D. Preparation and flame retardant property of nano-aluminum hydroxide foam for pre-venting spontaneous coal combustion. Fuel 2021, 304, 121494. https://doi.org/10.1016/j.fuel.2021.121494.
- DAZZEON. Available online: http://www.dazzeon.com/en/firesavior/ (accessed on 5 October 2022).
- CN114748828A. Efficient Environment-Friendly NP-Foam Extinguishing Agent and Preparation Method Thereof. 2022. Available online: https://patents.google.com/patent/CN114748828A/en (accessed on: 5 October 2022).
- Mosina, K.S.; Nazarova, E.A.; Vinogradov, A.V.; Vinogradov, V.V.; Krivoshapkina, E.F.; Krivoshapkin, P.V. Alumina Nano-particles for Firefighting and Fire Prevention. ACS Appl. Nano Mater. 2020, 3, 4386−4393. https://dx.doi.org/10.1021/acsanm.0c00506.
- Martin, T.J. Fire-Fighting Foam Technology. In Foam Engineering: Fundamentals and Applications; John Wiley & Sons: Chichester, UK, 2012; pp. 411−457.
- Zhou, R.; Dou, X.; Lang, X.; He, L.; Liu, J.; Mu, S. Foaming Ability and Stability of Silica Nanoparticle-Based Triple-Phase Foam for Oil Fire Extinguishing: Experimental. Soft Mater. 2018, 16, 327−338. https://doi.org/10.1080/1539445X.2018.1518878.
- Vinogradov, A.V.; Kuprin, D.S.; Abduragimov, I.M.; Kuprin, G.N.; Serebriyakov, E.; Vinogradov, V.V. Silica Foams for Fire Prevention and Firefighting. ACS Appl. Mater. Interfaces 2016, 8, 294−301. https://doi.org/10.1021/acsami.5b08653.
- Carn, F.; Colin, A.; Pitois, O.; Vignes-Adler, M.; Backov, R. Foam Drainage in the Presence of Nanoparticle−Surfactant Mix-tures, Langmuir 2009, 25, 14, 7847–7856. https://doi.org/10.1021/la900414q.
- Binks, B.P.; Rodrigues, J.A. Enhanced stabilization of emulsions due to surfactant-induced nanoparticle flocculation. Langmuir 2007, 23, 7436–7439. https://doi.org/10.1021/la700597k.
- Li, X.; Guo, R.; Qian, X. Research on the influence of wollastonite fibers on the stability of foam extinguishment agent and its effect on the extinguishing efficiency of pool fire. Fire Mater. 2020, 1–11. https://doi.org/10.1002/fam.2908.
- Hill, C.; Eastoe, J. Foams: From nature to industry. Adv. Colloid Interface Sci. 2017, 247, 496–513. http://dx.doi.org/10.1016/j.cis.2017.05.013.
- SERDP-ESRCP. Innovative Nano-Encapsulated Ionic Liquid-Based Surfactants for Fluorine-Free Fire Extinguishing Foams, WP20-1539. Available online: https://serdp-estcp.org/projects/details/44074bfa-601f-48c2-8250-3305ce801082 (accessed on 5 October 2022).
- SERDP-ESRCP. Innovative Nano-Encapsulated Ionic Liquid Based Surfactants for PFAS-Free Fire Extinguishing Foams, WP18-1597. Available online: https://serdp-estcp.org/projects/details/0e41dbfb-f716-408e-bf3c-5e443c43ba0e (accessed on 5 October 2022).
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The Flame-Retardant Mechanisms and Preparation of Polymer Composites and Their Potential Application in Construction Engineering. Polymers 2022, 14, 82; https://doi.org/10.3390/polym14010082.
- Maguire, J.F.; Woodcock, L.V. Thermodynamics of Tower-Block Infernos: Effects of Water on Aluminum Fires. Entropy 2020, 22, 14. http://dx.doi.org/10.3390/e22010014.
- Fire Safety Devices Pvt. Ltd. Wetting Agent. Available online: http://fcfsd.com/wetting-agents.html (accessed on 5 October 2022).
- Dali, F.A.; Shidlovsky, G.L.; Khasikhanov, M.S.; Zalaev, R.U.; Tagirova, P.R.; Saidulaev, S.S.; Masaeva, L.M.; Erzhapova, R.S. The use of carbon nanotubes in the fire extinguishing of oil and oil products. IOP Conf. Ser. Mater. Sci. Eng. 2020, 905, 012011. https://doi.org/10.1088/1757-899X/905/1/012011.
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 435873. https://doi.org/10.1155/2012/435873.
- Toropov, D.; Ivanov, A.; Dali, F.; Perlin, A.; Lebedev, A.; Shidlovsky, G. Extinguishing characteristics of water suspensions with carbon nanostructures at extinguishing liquid hydrocarbons fires (oil and gas industry). Fire Prot. Saf. Sci. J. 2019, 13, 2231. https://doi.org/10.17423/delta.2019.13.1.55.
- CN109966688B. High-Molecular Hydrogel Fire Extinguishing Agent and Preparation Method Thereof. 17 August 2019.
- SMH SISTEMAS. Fire Suppression System by Water Mist. Available online: https://www.smh.com.br/en/fire-suppression-system-by-water-mist/ (accessed on 5 October 2022).
- Liu, Y.P.; Wang, X.S.; Liu, T.; Ma, J.; Li, G.C.; Zhao, Z.H. Preliminary study on extinguishing shielded fire with water mist. Process Saf. Environ. Protect. 2020, 141, 344–354. https://doi.org/10.1016/j.psep.2020.05.043.
- Jiang, H.; Bi, M.; Huang, L.; Zhou, Y.; Gao, W. Suppression mechanism of ultrafine water mist containing phosphorus com-pounds in methane / coal dust explosions. Energy 2022, 239, 121987. https://doi.org/10.1016 / j.energy.2021.121987.
- Kudo, T.; Sekiguchi, K.; Sankoda, K.; Namiki, N.; Nii, S. Effect of ultrasonic frequency on size distributions of nanosized mist generated by ultrasonic atomization. Ultrason. Sonochem. 2017, 37, 16–22. https://doi.org/10.1016 / j.ultsonch.2016.12.01.
- Linteris, G.T.; Rumminger, M.D.; Babushok, V.I. Catalytic inhibition of laminar flames by transition metal compounds. Prog. Energy Combust. Sci. 2008, 34, 288–329. https://doi.org/10.1016/j.pecs.2007.08.002.
- Tianwei, Z.; Hao, L.; Zhiyue, H.; Zhiming, D.; Yong, W. Active substances study in fire extinguishing by water mist with potassium salt additives based on thermoanalysis and thermodynamics. Appl. Therm. Eng. 2017, 122, 429–43838.
- Dombrovsky, L.A.; Dembele, S.; Wen, J.X. A simplified model for the shielding of fire thermal radiation by water mists. Int. J. Heat Mass Transf. 2016, 96, 199–209. http://dx.doi.org/10.1016/j.ijheatmasstransfer.2016.01.028.
- Takahashi, F. Fire blanket and intumescent coating materials for failure resistance. MRS Bull. 2021, 46, 429–434. https://doi.org/10.1557/s43577-021-00102-7.
- WowZone Buyer’s Protection. FlameCape™ Fire Blanket. Available online: https://wowzonegadgets.com/products/flamecape-fire-blanket (accessed on 5 October 2022).
- Mulukutla, R.S.; Malchesky, P.S.; Maghirang, R.; Klabunde, J.S.; Klabunde, K.J.; Koper, O. Metal Oxide Nanoparticles for Smoke Clearing and Fire Suppression. US20080210444A1. 4 September 2010.
